Chapter 64 Genetic Diseases
Short QT Syndrome
Historical Context
It has long been recognized that long QT syndrome (LQTS) is a clinical entity with a significant risk of SCD. When analyzing retrospective Holter monitor strips, Algra et al first recognized that short Q-T intervals had an increased risk of SCD. Gussak and associates were the first to propose SQTS as a unique clinical entity in a case series of three patients from one family with similar electrocardiogram (ECG) findings in the setting of atrial and ventricular arrhythmias.1 In 2003, the definitive link was demonstrated between SQTS and SCD. Gaita et al studied several members of two families who exhibited Q-T intervals less than 280 ms and presented with syncope, palpitations, and a family history of SCD.2
Pathophysiology
Furthermore, the shortening of the refractory period is heterogeneous within the myocardial cells, leading to nonuniform dispersion of refractoriness.3 This, in turn, may lead to VF through the mechanism of wavebreak through re-entry initiation, wavelet formation and, ultimately, fibrillation.3–5
Molecular Genetics
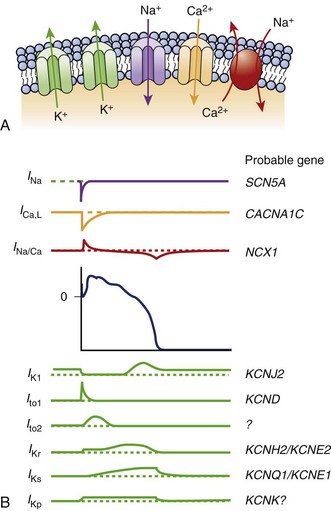
The KCNH2 gene, or HERG, encodes a transmembrane protein responsible for the rapidly activating delayed rectifier potassium channel IKr. Two missense mutations have been described that lead to “gain in function” and shortening of the APD leading to SQT1.6,7 This potassium channel is the most commonly implicated in LQTS as well.
The KCNQ1 gene encodes the ion channel responsible for the slowly activating delayed rectifier potassium channel IKs. A missense mutation in the KCNQ1 gene results in accelerated activation kinetics consistent with a gain of function in the outward current. This mutation also leads to shortening of the APD and is considered the sporadic form of SQTS, called SQT2.8,9
The KCNJ2 gene encodes for a protein responsible for the inward potassium rectifier current (IK1). A mutation in this gene generates electrical currents that do not decrease to the extent of normal potassium channels. This corresponds to the end of phase 3 of repolarization, leading to acceleration of late repolarization, thereby shortening the APD. This mutation leads to a unique ECG finding of asymmetric tall T waves with a rapid descending portion that is now called SQT3.10
Antzelevitch et al were the first to report loss-of-function mutations in genes encoding the cardiac L-type calcium channel to be associated with a familial SCD, in which a Brugada syndrome phenotype is combined with shorter than normal Q-T intervals. Among 82 probands with a clinically robust diagnosis of Brugada syndrome in the present registry, 6% (5 patients) presented with a shorter than normal Q-T interval, specifically mutations in CACNA1C and CACNB2b. Although the QTc intervals (330 to 360 ms) are longer than those encountered in KCNH2 and KCNQ1 mutations, they do overlap within the range found in patients with KCNJ2 mutations (SQT3) (Figure 64-1).11
Clinical Manifestations
SQTS has been characterized by major and minor cardiac events or symptoms. Major events include SCD, syncope, and VF. Minor events include palpitations, lightheadedness or dizziness, and paroxysmal AF. SCD is the most frequent first symptom and first clinical presentation. Most patients have a significant family history of SCD, occurring in relatives with a variable age distribution, ranging from 3 months to 70 years. The mean age of diagnosis is 30 years.12 It has been suggested that sudden infant death syndrome may, in some cases, be attributed to SQTS.12,13 Paroxysmal AF was first reported in 2000 as being linked to SQTS in a young woman who developed rapid AF during surgery. She subsequently underwent cardioversion to sinus rhythm that uncovered a Q-T interval of 280 ms. It was found that she had multiple family members with paroxysmal AF in the setting of short Q-T intervals.14 It is vital to exclude SQTS in a young patient with lone AF. SCD was definitively linked to SQTS in a case series in which five of six members were inducible for VF during EPS, and all six received an implantable cardioverter-defibrillator (ICD).2
The classic sign on ECG is a very short Q-T interval. A cutoff of 320 ms should raise suspicion for SQTS because the two families first identified by Gaita et al had Q-T intervals less than 280 ms.2 The T waves are tall, peaked, and symmetrical except in patients with the form of SQTS involving a mutation in the KCNJ2 gene (SQT3).10 The T wave is upright, and the interval of the T wave is not usually prolonged. The ST segment is often noted to be very short or completely absent. An appearance of a U wave has been reported in a few cases. Occasionally, the PR segment may be depressed, consistent with abnormal atrial repolarization.15 A slight reduction in the Q-T interval accompanies a physiological increase in the heart rate, although it can often be difficult to make a diagnosis of SQTS when the heart rate is above 100 beats/min. It is important to exclude other potential etiologies of acquired short Q-T interval, such as sinus tachycardia, hyperthermia, hypocalcemia, acidosis, hyperkalemia, and digoxin therapy.16
EPS uniformly reveals short atrial and ventricular refractory periods with easily inducible AF and VF by programmed stimulation. In a study by Giustetto et al, 18 patients with SQTS underwent EPS to determine effective refractory periods and inducibility of VT or VF. The effective refractory period (ERP), determined from the apex of the right ventricle at a drive cycle length of 500 to 600 ms, varied between 140 and 180 ms. The ERP from the high right atrium with drive cycle length of 600 ms varied between 120 and 180 ms. The significance of ventricular programmed stimulation as a risk stratifier in patients with inherited channelopathies is unclear. In the study by Giustetto et al, VF was induced in only 11 of 18 patients, of whom only three had a prior history of resuscitated cardiac arrest. Only three of six patients with prior documented VF could be induced by EPS.12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree