Fever after transcatheter aortic valve implantation (TAVI) is common and may result in extensive workup, treatment with broad-spectrum antibiotics, and prolonged hospitalization. Despite these consequences, the prevalence and nature of fever after TAVI and whether cases of fever could be attributed to an infectious origin have not been studied thoroughly to date. We conducted an observational retrospective analysis of 148 consecutive patients undergoing percutaneous transfemoral TAVI at the Tel-Aviv Medical Center. All patients were treated with antibiotic prophylaxis using first- or second-generation cephalosporins (or vancomycin upon a β-lactam allergy) on the procedure day. Medical and nursing records were reviewed for the occurrence, extent, and origin of fever. Laboratory databases were screened for positive cultures. Fever ≥37.5°C occurred in 66 patients (47%) and ≥38.0°C in 27 patients (19.4%). Most febrile episodes ≥38.0°C were of short duration, lasting <8 hours (59.3%, n = 16), and occurred in the first 48 hours after procedure (74%, n = 22). Bacteremia was found in 2 cases and urinary tract infection in 3 other cases; most pathogens isolated were resistant to prophylactic antibiotic regimen. Unlike prolonged fever, a short febrile episode was not associated with an extended hospital stay or with increased 30-day mortality rate after TAVI. In conclusion, fever after TAVI occurs frequently and may represent a noninfectious inflammatory response as it rarely associates with a documented bacterial infection. Therefore, deferring antibiotic therapy in an otherwise well post-TAVI patient with a short febrile episode should be considered, whereas prolonged and high-grade fever warrants further workup and empirical antibiotic therapy.
Transcatheter aortic valve implantation (TAVI) for the treatment of severe aortic stenosis is an emerging intervention offered for patients at high risk for cardiac surgery. Thousands of TAVI procedures are performed annually worldwide, and it is now routinely performed in many centers. Fever after TAVI is not infrequent and poses a clinical dilemma to cardiologists and infectious disease specialists. Although TAVI is considered a minimally invasive procedure, protective barriers are breached during its course by vascular accesses, urinary catheter, sedation, and sometimes intubation. This may lead to local and bloodstream infections, endangering the patients with early prosthetic valve endocarditis. In addition, similarly to other endovascular procedures, febrile episodes after TAVI may represent a noninfectious inflammatory response to rupture of heavily calcified plaques during valve deployment, valve material, contrast media, and hematomas. The development of fever in a post-TAVI patient may result in extensive workup, treatment with wide-spectrum antibiotics, and prolonged hospitalization. Because TAVI is a relatively new procedure, it is of major importance to establish risk stratification strategies directed at patients likely to be febrile due to sepsis, for whom rigorous workup and intervention are warranted versus low-risk patients for whom watchful vigilance may be beneficial. We report our experience comprehensively describing the prevalence of post-TAVI fever, its patterns, related infections, and presumed origin.
Methods
Data of 148 consecutive patients undergoing percutaneous transfemoral TAVI at the Interventional Cardiology Unit of the Tel-Aviv Medical Center, Tel-Aviv, Israel, from March 2009 to June 2012 were analyzed. Patients were recruited during their participation in the Tel-Aviv Angiography Prospective Study described in detail elsewhere. Informed consent was obtained as approved by the institutional ethics committee.
All patients received periprocedural (24 hours) antibiotic prophylaxis with a first- or a second-generation cephalosporin (or vancomycin upon a β-lactam allergy). Patients were either deeply sedated or intubated (first 10 cases only). A transesophageal echocardiographic probe was placed in the esophagus, urinary catheter was inserted, peripheral venous access was used for drug and fluid delivery, and a peripheral arterial sheath was placed for blood pressure monitoring. During the procedure, an 18Fr delivery sheath was placed into the femoral artery in all patients, and a temporary pacemaker was placed in the right ventricle through a central venous catheter. After prosthesis implantation, all patients were transferred to the cardiac intensive care unit where vital signs were recorded at least 3 times daily. In the absence of a specific indication, temporary pacemaker, urinary catheter, and arterial line were withdrawn 24 hours after procedure. Fever measurement ≥38.0°C prompted peripheral line extraction, 2 separate sets of blood cultures, and urinary analysis and culture. Infectious disease specialists were consulted regarding every febrile patient, and after culture attainment, empirical broad-spectrum antibiotic treatment was started at their discretion. Daily surveillance of the infectious diseases unit was conducted until fever resolution.
Clinical and laboratory data were extracted from medical and nursing records. All microbiologic samples were reviewed using laboratory computerized databases and analyzed by an infectious disease specialist. Fever measurements (≥37.5°C) within the first week after TAVI were collected, differentiating between patients with a single febrile episode and those with prolonged fever. Clinical outcomes including periprocedural complications, hospital length of stay, and functional class at 1 month after TAVI and mortality at 1, 6, and 12 months were assessed. Data were analyzed using SPSS software (SPSS, Chicago, Illinois). Different analyses were performed for febrile versus nonfebrile patients and for patients with prolonged fever (>8 hours) versus nonfebrile and short-duration fever.
Results
Our final cohort included 140 consecutive patients undergoing percutaneous transfemoral TAVI at our institution (65% women, n = 91), with a mean age of 83.7 years (range 69 to 94, SD 5). Eight patients were excluded because of the lack of clinical data. Similar to other TAVI cohorts, a baseline high prevalence of co-morbidities and previous vascular disease was observed ( Table 1 ). Because our initial experience with TAVI procedure was performed using the Medtronic CoreValve prosthesis (Medtronic, Minneapolis, Minnesota), nearly all patients in the present cohort received this prosthesis (137 of 140), whereas the other 3 patients received an Edwards SAPIEN prosthesis (Edwards Lifesciences, Irvine, California).
| Variable | Total Cohort, n = 140 (%) | Fever | p Value | |
|---|---|---|---|---|
| No, n = 113 (%) | Yes, n = 27 (%) | |||
| Age, mean ± SD, (range), yrs | 83.7 ± 5 (69–94) | 83.8 ± 5 | 83.4 ± 5 | 0.89 |
| Men | 49 (35) | 36 (32) | 13 (48) | 0.12 |
| Diabetes mellitus | 41 (29) | 34 (30) | 7 (26) | 0.81 |
| Dyslipidemia ∗ | 117 (84) | 93 (82) | 24 (89) | 0.56 |
| Hypertension † | 116 (83) | 94 (83) | 22 (82) | 0.78 |
| Smoker | 29 (21) | |||
| Height, mean ± SD (range), cm | 162 ± 9 (145–183) | 161 ± 9 | 163 ± 9 | 0.65 |
| Weight, mean ± SD (range), kg | 71 ± 15 (40–105) | 70 ± 14 | 75 ± 16 | 0.26 |
| Body mass index, mean ± SD (range), (kg/m 2 ) | 27 ± 5 (19–42) | 27 ± 4 | 28 ± 5 | 0.16 |
| Peripheral vascular disease | 20 (14) | 16 (14) | 4 (15) | NS |
| Previous stroke | 10 (7) | 8 (7) | 2 (7) | NS |
| Congestive heart failure | 64 (46) | 47 (42) | 17 (63) | 0.054 |
| History of coronary artery disease ‡ | 78 (56) | 60 (53) | 18 (67) | 0.28 |
| Previous coronary bypass | 21 (15) | 18 (16) | 3 (11) | 0.76 |
| Normal or nonobstructive coronary disease | 67 (48) | |||
| 1-Vessel disease | 28 (20) | |||
| 2-Vessel disease | 17 (12) | |||
| 3-Vessel disease and/or left main disease | 28 (20) | |||
| Chronic atrial fibrillation | 27 (19) | 20 (18) | 7 (26) | 0.41 |
| Ejection fraction, mean ± SD (range), (%) | 56 ± 7 (30–69) | 56 ± 7 | 55 ± 8 | 0.62 |
| EuroSCORE, mean ± SD (range) | 27 ± 13 (5–63) | 27 ± 13 | 26 ± 14 | 0.75 |
∗ Low-density lipoprotein >100 mg/dl, high-density lipoprotein <40 mg/dl, or chronic treatment with lipid-lowering medication.
† Blood pressure measurements >140/90 mm Hg or treatment with chronic medication with the intention of lowering blood pressure.
‡ Previous diagnosis and/or treatment of epicardial artery narrowing of >70%.
Within 7 days after TAVI, fever ≥37.5°C occurred in 66 patients (47%) and fever ≥38.0°C occurred in 27 patients (19.4%). Patient baseline characteristics were similar for those with or without fever ≥38.0°C ( Table 1 ), and no preprocedural clinical predictors for fever were found. Of these 27 febrile patients, 11 (40.7%) had at least 2 measured temperature spikes and fever lasting >8 hours ( Figure 1 ). Most febrile episodes occurred in the first 48 hours after the procedure (74%, 20 of 27 episodes).
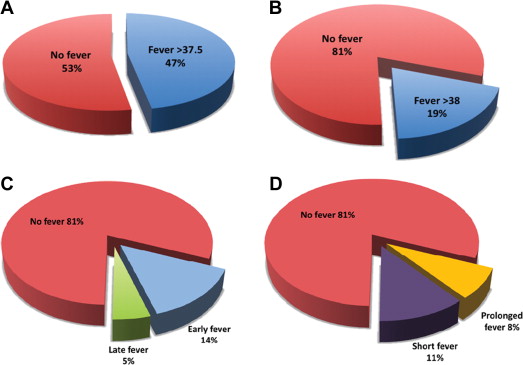
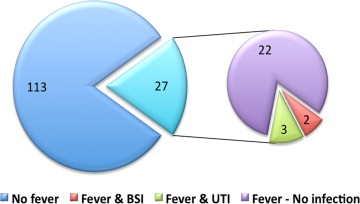
Empirical broad-spectrum antibiotic was initiated in 81.5% (n = 22) of febrile patients, and in all cases when fever was prolonged (>8 hours of fever). Within the first week after TAVI, documented infections were found in 5 febrile patients (18.5% and 3.6% of febrile patients and all patients, respectively, Figure 2 ). Bloodstream infection was documented in 2 febrile patients, both had prolonged and high-grade fever (≥38.0°C), and both pathogens isolated were resistant to the standard prophylactic regimen ( Enterococcus faecalis and extended-spectrum β-lactamase producing Klebsiella pneumoniae ). Urinary pathogens were isolated from 3 other febrile women, none with an indwelling urinary catheter, yet only one had prolonged fever. These isolates were mostly resistant to the prophylactic antibiotics used. Identified infections were not related to increased mortality.
The primary outcome analysis, combining all post-TAVI febrile episodes (short and prolonged duration), demonstrated that the occurrence of fever was not associated with an increased 30-day mortality rate or combined outcome score (Valve Academic Research Consortium-2; Table 2 ). Of all other outcomes assessed, only early postprocedural stroke and pericardial tamponade were associated with a febrile event of any duration.
| Variable | Fever | p Value | |
|---|---|---|---|
| Yes, n = 27 (%) | No, n = 113 (%) | ||
| Mortality (mo) | |||
| 1 | 2 (7) | 2 (2) | 0.17 |
| 6 | 3 (11) | 8 (7) | 0.44 |
| 12 | 5 (19) | 16 (14) | 0.55 |
| Postprocedural complications | |||
| Myocardial infarction | 0 | 0 | 1 |
| Tamponade | 2 (7) | 0 | 0.036 |
| Cardiogenic shock | 1 (4) | 2 (2) | 0.48 |
| Respiratory failure | 0 | 2 (2) | 1 |
| Cerebrovascular accident | 2 (7) | 0 | 0.036 |
| Major bleeding | 0 | 3 (3) | 1 |
| Major vascular complication | 2 (7) | 1 (1) | 0.09 |
| Minor vascular complication | 3 (11) | 15 (13) | 1 |
| Combined safety end point | 4 (15) | 5 (4) | 0.07 |
| Average functional class at 30 days | 1.22 | 1.22 | 0.94 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


