Dabigatran is a univalent low-molecular-weight direct thrombin inhibitor that has been developed as an alternative to vitamin K antagonists (VKAs). However, uncertainty remains regarding dabigatran’s safety profile with respect to bleeding. Our objective was to compare the risk of bleeding and all-cause mortality of dabigatran with that of VKAs in a systematic review and meta-analysis of randomized controlled trials (RCTs). We systematically searched MEDLINE, Embase, and the Cochrane Library of clinical trials to identify RCTs comparing the bleeding risk of dabigatran (150 mg twice daily) with that of VKAs. Included RCTs had treatment duration ≥90 days and were published in English or French. Data were meta-analyzed using random-effects models. Five RCTs (n = 20,332) were included in our systematic review. Study populations consisted of patients with atrial fibrillation (n = 18,615) and venous thromboembolism (n = 7,998). When data were pooled across the 4 RCTs (n = 17,466) without overlapping populations, dabigatran was not associated with an increased risk of major bleeding compared with VKAs (relative risk [RR] 0.92, 95% confidence interval [CI] 0.81 to 1.05). Dabigatran was associated with a decreased risk of intracranial bleeding (RR 0.40, 95% CI 0.27 to 0.59) but an increased risk of gastrointestinal bleeding (RR 1.51, 95% CI 1.23 to 1.84). Dabigatran was also associated with a trend toward decreased all-cause mortality (RR 0.90, 95% CI 0.80 to 1.01). In conclusion, results suggest that dabigatran has a favorable safety profile with respect to bleeding compared with VKAs.
Dabigatran is a univalent low-molecular-weight direct thrombin inhibitor. Directly inhibiting thrombin blocks the conversion of fibrinogen to fibrin, thus diminishing thrombus growth. Dabigatran has been shown to be efficacious in the treatment of venous thromboembolism (VTE) and prevention of stroke and death in patients with atrial fibrillation (AF). However, concerns remain regarding the potential risk of bleeding associated with its use. We therefore conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to compare the risk of bleeding and mortality of dabigatran with that of vitamin K antagonists (VKAs).
Methods
We conducted this systematic review and meta-analysis following a prespecified protocol and we report it according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
We systematically searched MEDLINE, Embase, and Cochrane Library of clinical trials databases from inception to June 27, 2013 to identify all RCTs comparing dabigatran with VKAs. The search, which is described in detail in on-line Appendix 1, A to C , was conducted using the following terms: “dabigatran,” “dabigatran etexilate,” “Pradaxa,” “BIBR 953,” “BIBR 1048,” “thrombin inhibitor,” “direct thrombin inhibitor,” “vitamin K antagonist,” “warfarin,” “acenocoumarol,” and “low molecular weight heparin.” The MEDLINE and Embase searches (on-line Appendix 1, A and B ) were limited to RCTs using a modified version of the McMaster RCT hedge. All searches were limited to studies published in English or French. In addition, we hand searched the references of relevant trials and previous reviews to identify additional RCTs not included in our electronic search.
Trials were included if they (1) were RCTs comparing 150 mg twice daily of dabigatran with a VKA or low-molecular-weight heparin, (2) included adults aged ≥18 years, and (3) included a treatment duration of ≥90 days. Inclusion was restricted to RCTs involving 150 mg twice daily of dabigatran as this is the clinically recommended dosage. Reviews, editorials, and letters to the editor were excluded from our study. In addition, trials were excluded if not published in English or French.
Data from RCTs that met our inclusion criteria were independently extracted by 2 reviewers using a standardized, pilot-tested, data extraction form. Disagreements were resolved by consensus or by a third reviewer when necessary. For trials that included multiple dabigatran treatment arms, only data from the 150 mg twice daily of dabigatran arm were extracted. Data extracted included study and patient characteristics, as well as outcomes related to bleeding and death. Basic study characteristics included the population, country where the trials were conducted, number of centers, and randomization procedures. Baseline patient characteristics included age, gender, baseline co-morbidities, and previous VKA use. Outcomes extracted included major or clinically relevant nonmajor bleeding, any bleeding, major bleeding, fatal bleeding, bleeding resulting in a fall in hemoglobin ≥2 g/dl, intracranial bleeding, intraocular bleeding, retroperitoneal bleeding, intra-articular bleeding, gastrointestinal (GI) bleeding, and all-cause mortality. Bleeding definitions used in each RCT are reported in on-line Appendix 2 .
Trial quality was assessed using the Cochrane Collaboration’s tool for assessing risk of bias (on-line Appendix 3 ). The tool comprises the following domains: sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective outcome reporting; and other potential sources of bias. Each domain was given a score of “high,” “low,” or “unclear” independently by 2 reviewers, and disagreements were resolved by consensus or by a third reviewer when necessary.
We used DerSimonian and Laird random-effects models to pool data across included RCTs and estimate relative risks (RRs) and the corresponding 95% confidence intervals (CIs). In our primary analyses, data were pooled using count data as data were most frequently reported in this manner. All analyses were stratified by study population (AF vs VTE), with a summary estimate obtained by pooling data across populations. I 2 values were calculated to estimate the amount of between-study heterogeneity that was present. Publication bias was assessed by way of visual inspection of a funnel plot and using Egger’s test for small-study effects. All analyses were conducted using Stata, version 11.2 (StataCorp LP, College Station, Texas).
Results
Our electronic literature search generated 1,583 potentially eligible publications ( Figure 1 ). After the removal of 404 duplicates, the titles and abstracts of the remaining 1,179 publications were screened, for which the full texts were retrieved for 54 reports. After a detailed review of these reports, 5 met all inclusion criteria and were included in our systematic review.
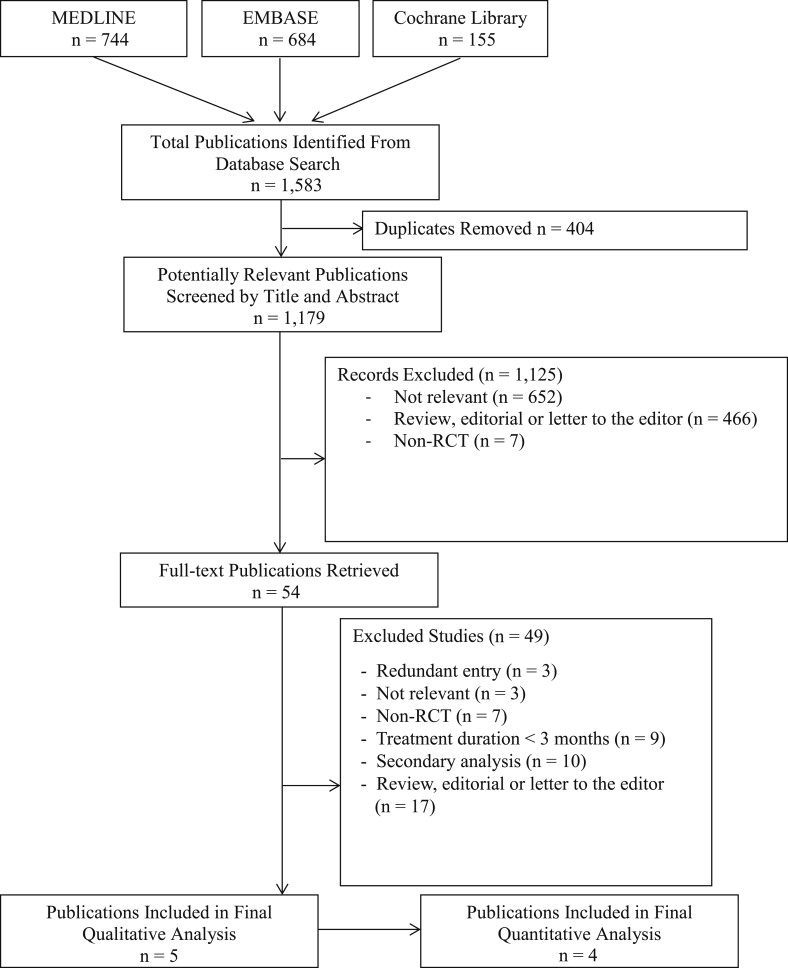
The 5 multicenter RCTs included a total of 20,332 patients ( Table 1 ). Sample size ranged from 502 to 18,113 patients. The Secondary Prevention of VTE (RE-MEDY) trial was partly composed of patients from the previous Efficacy and Safety of Dabigatran Compared to Warfarin for 6 Month Treatment of Acute Symptomatic VTE (RE-COVER) and Phase III Study Testing Efficacy & Safety of Oral Dabigatran Etexilate vs Warfarin for 6 Month Treatment for Acute Symptomatic VTE (RE-COVER II) trials (17% and 4%, respectively). Thus, it was included in the systematic review but not in the meta-analysis. In all RCTs, patients were predominantly men, with a mean age ranging from 55 to 71 years. Included patients were treated for AF (n = 18,615) or VTE (n = 7,998). Treatment and follow-up durations ranged from 84 to 730 days. In AF trials, the primary efficacy end points were thromboembolic events or a composite of stroke or systemic embolism. In the VTE trials, the primary efficacy end points were recurrent or fatal VTE. In both populations, safety outcomes included various bleeding and mortality outcomes. Safety end points were examined using as-treated analyses in all included RCTs except for the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, which used an intention-to-treat analysis.
| Characteristic | RE-LY , † | PETRO | RE-COVER II | RE-COVER | RE-MEDY | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DAB | VKA | DAB | VKA | DAB | VKA | DAB | VKA | DAB | VKA | |
| Total number of patients (n) | 6,076 | 6,022 | 166 | 70 | 1,280 | 1,288 | 1,282 | 1,282 | 1,433 | 1,433 |
| Study population | AF | AF | VTE | VTE | VTE | |||||
| Age (yrs; mean ± SD) | 71.5 ± 8.8 | 71.6 ± 8.6 | 70 ± 8.1 | 69 ± 8.3 | NR | NR | 55.0 ± 15.8 | 54.4 ± 16.2 | 55.4 ± 15.0 | 53.9 ± 15.3 |
| Women (%) | 36 | 36 | 18 | 15 | NR | NR | 41 | 40 | 39 | 38 |
| Drug discontinuation rate (%) | 15 | 10 | 6 | 2 | NR | NR | 16 | 14 | 19 | 19 |
| Length of treatment (days) | 730 | 84 | 180 | 180 | 180–1,095 § | |||||
| Length of follow-up (days) | 730 ‡ | 84 | 180 | 180 | 210–1,125 § | |||||
| Number of patients lost to follow-up | NR | NR | NR | NR | NR | NR | 9 | 6 | 2 | 6 |
| Percentage of time during which the INR was in therapeutic range | NA | 64 | NA | 57 | NA | NR | NA | 59 | NA | 65 |
| Previous use of VKA (%) ¶ | 50 | 48 | 100 | 100 | NR | NR | NR | NR | NR | NR |
∗ Table is stratified by study population and presented in decreasing order with respect to total sample size.
† Only patients in the 150-mg twice-daily arm were included in the systematic review and meta-analysis.
‡ Value is median length of follow-up. Others are lengths set by the protocol.
§ In the RE-MEDY trial, treatment duration ranged from 6 to 36 months, with follow-up lasting 30 days beyond the treatment period.
All included RCTs had a low risk of bias as defined by the Cochrane tool for evaluating the risk of bias (on-line Appendix 3 ). All RCTs were double-blind except for RE-LY, which was open label with respect to warfarin. However, outcomes in this trial were evaluated by assessors who were blind to treatment status, and it was thus considered to have a low risk of bias for the blinding domain. Double-blinding was achieved by having a computer programed point-of-care coagulometer to measure true international normalized ratios and sham international normalized ratios based on randomized assignments. Allocation concealment was done in a low-risk fashion in all trials except for the Prevention of Embolic and Thrombotic Events in Patients with Persistent AF (PETRO) trial, which did not describe its allocation concealment methods, resulting in a rating of “unclear.” Overall, the trials did not appear to have any other sources of bias. However, with the RE-COVER II trial only currently available as a conference abstract, a thorough assessment of potential sources of bias was not possible. This trial was therefore assigned a rating of “unclear” for this domain.
Bleeding outcomes were broken down into various subcategories in the included trials ( Tables 2 and 3 ). In both RE-COVER and RE-MEDY, patients randomized to dabigatran had a substantially lower risk of major or clinically relevant nonmajor bleeding events than those randomized to VKAs. In contrast, the results from PETRO were inconclusive with respect to this outcome due to wide 95% CIs. All trials indicated that patients randomized to dabigatran had a lower risk of any bleeding than those randomized to VKAs, although estimates from PETRO were again inconclusive due to sparse data. In all trials, the incidence of major bleeding was lower in patients randomized to dabigatran than in those randomized to VKAs, but none of the individual RCTs reached statistical significance. The only 2 trials assessing the risk of GI bleeding (RE-LY and RE-COVER) found that patients randomized to dabigatran had a consistently and statistically significant higher risk than those randomized to VKAs (RR 1.50, 95% CI 1.20 to 1.89 and RR 1.51, 95% CI 1.00 to 2.30, respectively). In contrast, dabigatran patients in RE-COVER had a lower risk of both intracranial bleeding (RR 0.41, 95% CI 0.28 to 0.60) and intra-articular or intramuscular bleeding (RR 0.29, 95% CI 0.13 to 0.65). Trial-specific all-cause mortality data were inconclusive in all trials except RE-LY, where a trend toward lower mortality was observed with dabigatran (RR 0.89, 95% CI 0.79 to 1.01).
| Outcome | Patients With AF | Patients With VTE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RE-LY | PETRO | RE-COVER II | RE-COVER | RE-MEDY | ||||||
| DAB (n = 6,076) | VKA (n = 6,022) | DAB (n = 166) | VKA (n = 70) | DAB (n = 1,280) | VKA (n = 1,288) | DAB (n = 1,274) | VKA (n = 1,265) | DAB (n = 1,430) | VKA (n = 1,426) | |
| Major/clinically relevant nonmajor bleeding events | NR | NR | 9 (5.4) | 4 (5.7) | NR | NR | 71 (5.6) | 111 (8.8) | 80 (5.6) | 145 (10.2) |
| Major bleeding | 375 (6.2) | 397 (6.6) | 0 | 0 | 15 (1.2) | 22 (1.7) | 20 (1.6) | 24 (1.9) | 13 (0.9) | 25 (1.7) |
| Associated with fall in hemoglobin ≥2 g/dl | NR | NR | NR | NR | NR | NR | 20 (1.6) | 18 (1.4) | 9 (0.6) | 18 (1.3) |
| Fatal | NR | NR | NR | NR | NR | NR | 1 (0.1) | 1 (0.1) | 0 | 1 (0.1) |
| GI | 182 (3.0) | 120 (2.0) | NR | NR | NR | NR | 53 (4.2) | 35 (2.8) | NR | NR |
| Intracranial | 36 (0.6) | 87 (1.4) | NR | NR | NR | NR | 0 | 3 (0.2) | NR | NR |
| Intraocular | NR | NR | NR | NR | NR | NR | 8 (0.6) | 9 (0.7) | NR | NR |
| Intra-articular or intramuscular | NR | NR | NR | NR | NR | NR | 8 (0.6) | 27 (2.1) | NR | NR |
| Any bleeding | 1,977 (32.5) | 2,142 (35.6) | 15 (9.0) | 12 (17.1) | 200 (15.6) | 285 (22.1) | 205 (16.1) | 277 (21.9) | 277 (19.4) | 373 (26.2) |
| All-cause mortality | 438 (7.2) | 487 (8.1) | NR | NR | 25 (1.9) | 25 (1.9) | 21 (1.7) | 21 (1.7) | 17 (1.2) | 19 (1.3) |
∗ Table is stratified by study population and presented in decreasing order of total sample size.
| Outcome | Patients With AF | Patients With VTE | Pooled ‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RE-LY | PETRO | RE-COVER II | RE-COVER | RE-MEDY , § | |||||||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | I 2 | |
| Major/clinically relevant nonmajor bleeding events | NR | NR | 1.57 | 0.50–4.91 | NR | NR | 0.63 | 0.47–0.84 | 0.54 | 0.41–0.71 | NR | NR | NR |
| Major bleeding | 0.94 | 0.82–1.07 | 0.70 | 0.01–35.0 | 0.69 | 0.36–1.32 | 0.83 | 0.46–1.50 | 0.52 | 0.27–1.02 | 0.92 | 0.81–1.05 | 0.0 |
| Associated with fall in hemoglobin ≥2 g/dl | NR | NR | NR | NR | NR | NR | 1.12 | 0.59–2.11 | 0.50 | 0.22–1.11 | NR | NR | NR |
| Fatal | NR | NR | NR | NR | NR | NR | 0.99 | 0.06–15.9 | 0.33 | 0.01–8.15 | NR | NR | NR |
| GI | 1.50 | 1.20–1.89 | NR | NR | NR | NR | 1.51 | 1.00–2.30 | NR | NR | 1.51 | 1.23–1.84 | 0.0 |
| Intracranial | 0.41 | 0.28–0.60 | NR | NR | NR | NR | 0.14 | 0.01–2.76 | NR | NR | 0.40 | 0.27–0.59 | 0.0 |
| Intraocular | NR | NR | NR | NR | NR | NR | 0.88 | 0.34–2.28 | NR | NR | NR | NR | NR |
| Intra-articular or intramuscular | NR | NR | NR | NR | NR | NR | 0.29 | 0.13–0.65 | NR | NR | NR | NR | NR |
| Any bleeding | 0.91 | 0.87–0.96 | 0.53 | 0.26–1.07 | 0.71 | 0.60–0.83 | 0.74 | 0.63–0.87 | 0.71 | 0.61–0.83 | 0.77 | 0.64–0.93 | 81.0 |
| All-cause mortality | 0.89 | 0.79–1.01 | NR | NR | 1.01 | 0.58–1.74 | 1.00 | 0.55–1.82 | 0.90 | 0.47–1.72 | 0.90 | 0.80–1.01 | 0.0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


