We evaluated the incidence of conduction abnormalities and requirement for permanent pacemaker in patients undergoing transcatheter aortic valve implantation (TAVI) with the Edwards Sapien prosthesis. In 2009, >8,000 patients were treated with TAVI using 1 of the 2 commercialized models of bioprosthesis (Edwards Sapien, Edwards Lifesciences, Irvine, California; and CoreValve, Medtronic, Irvine, California). Occurrence of conduction abnormalities including complete atrioventricular block requiring permanent pacemaker has been reported after TAVI with the 2 models of valve, more frequently with the CoreValve. We analyzed standard 12-lead electrocardiograms of 69 consecutive patients in whom an Edwards Sapien prosthesis was successfully implanted. Electrocardiograms were examined before treatment, at day 1, and at 1-month follow-up. Heart rate, PR and QT intervals and QRS duration were measured and the presence of a first-, second-, or third-degree atrioventricular block was documented. There was a slight increase in heart rate and a discrete decrease in QT interval at day 1. These values had returned to baseline values at 1 month. There was no change in PR interval but a transitory increase in QRS duration was noted. Frequency of left bundle branch block increased from 14.5% at baseline to 27.5% at day 1 with a decreased incidence at day 30 (21.3%). Permanent pacemaker was required in only 3 patients (4.3%). In conclusion, in our experience, conductive disorders and requirement of a definitive pacemaker after implantation of an Edwards Sapien aortic bioprosthesis are infrequent. The physical properties of this prosthesis may explain this observation.
In 2002, the first transcatheter aortic valve implantation (TAVI) was performed by our group in a patient with severe aortic stenosis and cardiogenic shock. Several studies have confirmed the feasibility and safety of TAVI, and 7 years later >8,000 patients have benefited from TAVI worldwide. Two models of prosthesis are available: the balloon-expandable Edwards Sapien prosthesis (Edwards Lifesciences, Irvine, California) and the self-expandable CoreValve (Medtronic, Irvine, California), which were approved by the European standards authorities in 2007. Among the risks of the procedure, one frequently reported is the occurrence of intraventricular or atrioventricular (AV) conduction abnormalities that may require permanent pacing. Occurrence of this disorder is variable with the 2 models of prosthesis used, but has been shown to be higher with the CoreValve than with the Edwards Sapien prosthesis. In this context of increasing numbers of implantations worldwide, we sought to evaluate the incidence of conduction disturbances after implantation of the Edwards Sapien prosthesis.
Methods
From April 2006 to May 2009, 87 consecutive patients with severe aortic stenosis underwent implantation with an Edwards Sapien prosthesis at Rouen University Hospital (Rouen, France). They were included in 2 prospective European multicenter feasibility studies (Registry of EndoVascular Implantation of Valves in Europe [REVIVE] and Placement of AoRTic TraNscatheter valves trial (EUrope) [PARTNER-EU]) and thereafter in the European SOURCE postmarket registry. Inclusion criteria were an aortic valve area <1 cm 2 (<0.6 cm 2 /m 2 ) in symptomatic patients (New York Heart Association functional class ≥II) presenting with high or prohibitive risk for surgery as assessed by a logistic European System for Cardiac Operative Risk Evaluation score ≥20%, with an aortic annulus diameter measured by echocardiography from 19 to 24 mm.
Of the 87 patients, 18 were excluded from the study because 12 patients already had a permanent pacemaker (13.6%), 1 with severe aortic regurgitation after TAVI was converted to conventional surgery for valve replacement at day 1, 3 died within 24 hours, and 2 did not undergo implantation due to failure of femoroiliac catheterization. The other 69 patients were therefore included for evaluation of conduction disturbances and represent our study group. Patients’ mean age was 82.5 ± 7.8 years and 47.8% were women. The logistic European System for Cardiac Operative Risk Evaluation score was 28.1 ± 13.8%. Other baseline characteristics are presented in Table 1 .
| Age (years), mean ± SD | 82.5 ± 7.8 |
| Women | 33 (48%) |
| Diabetes mellitus | 13 (19%) |
| Arterial hypertension | 43 (62%) |
| Dyslipidemia | 36 (54%) |
| Previous stroke | 11 (16%) |
| Peripheral arterial disease | 14 (26%) |
| Previous thoracic radiotherapy | 9 (13%) |
| Porcelain aorta | 6 (9%) |
| Long-term respiratory insufficiency | 26 (39%) |
| Long-term renal insufficiency | 24 (35%) |
| Previous coronary bypass | 17 (25%) |
| Left ventricular ejection fraction (%) | 52.3 ± 16.6 |
| Size of aortic annulus (mm) | 21.4 ± 1.5 |
| Transfemoral/transapical route | 54 (78%)/15 (22%) |
| Valve 26 mm/23 mm | 37 (54%)/32 (46%) |
| Postprocedural aortic regurgitation (≤2/3/4) | 65 (94%)/4 (6%)/0 |
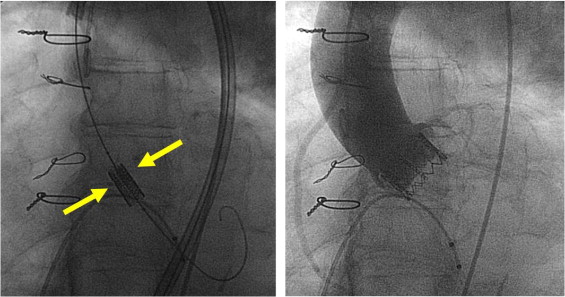
The device and procedures have been described in detail elsewhere. The Edwards Sapien prosthesis is made of bovine pericardium sutured onto a stainless-steel balloon-expandable stent. Two sizes are available: 23 and 26 mm with lengths of 14.1 and 16.1 mm, respectively ( Figure 1 ). Selection of the prosthesis size is based on measurement of the aortic annulus diameter by transthoracic or transesophageal echocardiography. The prosthesis can be implanted using the transfemoral retrograde or transapical approach. Balloon predilatation of the native valve (1 balloon inflation or 2 balloon inflations) is first performed using balloon sizes 2 mm smaller than the aortic annulus size, under rapid right ventricular pacing (200 to 220 beats/min) aimed at creating a transient ventricular tachycardia-induced cardiac standstill. Pacing is achieved using a temporary lead placed into the right ventricle through the femoral vein for transfemoral procedures and an epicardial electrode for the transapical route. Positioning and deployment of the prosthesis are accomplished in our center solely under fluoroscopic guidance without the help of online transesophageal echocardiography. The Edwards Sapien prosthesis was conceived to be implanted in the subcoronary position with no interference with the intraventricular septum and anterior mitral valve leaflet. The aortic valve plane that can be seen as a strip of calcium in the fluoroscopic monitors is used as a landmark. Prosthesis delivery is accomplished by manual balloon inflation under rapid ventricular pacing. After delivery, this strip of calcium should project at the equator of the stent ( Figure 2 ). A 3-lead electrocardiogram is continuously recorded during TAVI and, in the absence of conduction abnormalities during the procedure, the stimulation probe is withdrawn immediately after the transfemoral procedures and within 24 hours after the transapical procedures. Electrocardiogram is continuously recorded and stored during ≥24 hours after the procedures in the intensive or postoperative care unit.

We analyzed standard 12-lead electrocardiograms of all consecutive patients in whom the Edwards Sapien valve was successfully implanted before (baseline) and after TAVI at day 1 and at 1 month. Electrocardiographic (ECG) data studied included rhythm, heart rate (beats per minute), duration of PR interval (milliseconds) and of QRS complex (milliseconds), corrected QT interval (milliseconds), and the presence of a first-, second-, or third-degree AV block. The diagnostic criteria recommended by the World Health Organizational/International Society and Federation for Cardiology Task Force were used to define the presence of fascicular hemiblocks and left or right bundle branch block (LBBB or RBBB, respectively). Need for temporary or permanent pacing was documented. Per-procedural ECG changes were also assessed on continuous 3-lead ECG monitoring.
Continuous variables are presented as mean ± SD. Categorical variables are presented as frequencies and percentages. For continuous variables, comparisons between before and after the procedure and before the procedure and follow-up at 1 month were done using Student’s t test for normally distributed data, and chi-square test and Fisher’s exact test were used to compare categorical variables.
Results
TAVI was performed using the transfemoral approach in 54 patients (78.2%) and the transapical approach in 15 patients (21.8%). Mean hospital stay was 10.1 ± 6.5 days. Electrocardiograms were analyzed in all 69 patients at day 1 and in 64 of 69 patients at 1 month; 3 patients died within the interval and 2 ECG tracings were not available for interpretation. Clinical evaluation was obtained during follow-up in all cases. Survival at 1 month was 95.6%.
Before the procedure, most patients (79.7%) were in sinus rhythm, and this number was unchanged at day 1 (76.8% vs 79.7%, p = 0.57) and at 1 month (76.6%, p = 0.41). Transient atrial fibrillation occurred after the transapical procedure in 2 patients. Compared to baseline, there was a slight increase of heart rate at day 1 (79.3 ± 13.7 vs 74.4 ± 13.7 beats/min, p = 0.002), with a return to baseline at 1 month (76.3 ± 15.8 beats/min, p = 0.22). A significant increase in QRS duration was noted at day 1 (117.6 ± 27.9 vs 113.2 ± 27.4 ms, p = 0.015), with a return to baseline at 1 month (113.1 ± 25.6 ms, p = 0.7). QT interval was slightly shorter at day 1 (403.7 ± 48 vs 426.7 ± 41.3 ms, p = 0.0002), with a return to baseline at 1 month (419.9 ± 47.8 ms, p = 0.16).
Abnormalities in conduction are presented in Table 2 . There was no significant increase in PR interval at day 1 (197.7 ± 43.6 vs 194.4 ± 40.2 ms, p = 0.62) and at 1 month (194.9 ± 36.5 ms, p = 0.7) and frequency of first-degree AV block remained unchanged over the study period. Frequency of LBBB significantly increased from 14.5% (n = 10) at baseline to 27.5% (n = 19) at day 1 (p = 0.015). These 9 new LBBBs occurred in the course of 7 transfemoral and 2 transapical procedures. It appeared in 4 cases after balloon predilatation, in 3 cases after valve implantation, and in 2 transapical procedures the timing could not be assessed. At day 30, LBBB persisted in only 3 of these 9 patients. Left fascicular hemiblock (3 patients) or incomplete LBBB (3 patients) was documented in the other 6 patients. Frequency of RBBB remained unchanged from baseline (10.1%) to day 1 and 1 month. No patient with RBBB at baseline developed AV block during the procedure or in the course of follow-up. A permanent pacemaker was required in 3 of 69 patients (4.3%). One patient (transfemoral approach) who had a normal electrocardiogram at baseline developed third-degree AV block immediately after balloon predilatation of the native valve. The patient remained pacer dependent at day 3 and permanent pacing was required. The second patient who had a LBBB (QRS duration 134 ms) at baseline developed atrial fibrillation during a transapical procedure. QRS duration was unchanged at day 1 (QRS duration 128 ms) and the patient was discharged at day 8 under amiodarone. At day 10, high-degree AV block was detected, leading to the indication of permanent pacing. At 1 month, the patient was not pacer dependent and QRS duration had decreased to 96 ms. The third patient who had a left anterior hemiblock at baseline developed a LBBB with first-degree AV block (PR interval 400 ms) immediately after implantation of the device (transfemoral approach). An electrophysiologic study was undertaken at day 7, which documented an HV interval of 94 ms leading to the indication of permanent pacing. The patient was no more pacer dependent at 1 month, in sinus rhythm with normal PR interval. The ratios/diameters of the Edwards valve/annulus diameter were 1.04, 1.09, and 1.3 in these 3 patients.
| Variables | Before Implantation | Day 1 After Implantation | Day 30 After Implantation | p Value | ||
|---|---|---|---|---|---|---|
| Before vs Day 1 | Day 1 vs 30 | Before vs Day 30 | ||||
| Sinus rhythm | 55 (80%) | 53 (77%) | 50 (77%) | 0.57 | 0.43 | 0.42 |
| Normal electrocardiogram | 20 (29%) | 14 (20%) | 11 (18%) | 0.07 | 0.21 | 0.04 |
| First-degree atrioventricular block | 22 (32%) | 18 (26%) | 20 (33%) | 0.27 | 0.1 | 0.33 |
| Left anterior hemiblock | 19 (28%) | 17 (25%) | 18 (29%) | 0.57 | 0.12 | 0.25 |
| Left posterior hemiblock | 1 (1.1%) | 1 (1.1%) | 1 (1.6%) | 0.1 | 0.24 | 0.27 |
| Left bundle branch block | 10 (14%) | 19 (28%) | 13 (21%) | 0.015 | 0.09 | 0.08 |
| Left incomplete block | 18 (26%) | 17 (25%) | 17 (28%) | 0.77 | 0.17 | 0.25 |
| Right bundle branch block | 7 (10%) | 7 (10%) | 6 (10%) | 1 | 0.14 | 0.18 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


