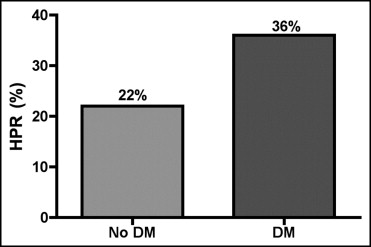
The effect of periprocedural platelet reactivity and clinical outcomes in diabetic patients taking clopidogrel and undergoing percutaneous coronary intervention (PCI) is unclear. The aim of the present study was to prospectively evaluate the influence of diabetes mellitus (DM) on platelet reactivity measured by the VerifyNow P2Y12 assay and on periprocedural outcomes in patients receiving clopidogrel and undergoing PCI. A total of 285 consecutive clopidogrel-treated patients undergoing elective PCI were included. Platelet function analysis was performed using the VerifyNow P2Y12 assay. High platelet reactivity (HPR) after clopidogrel was defined as a platelet reaction unit value ≥240. Cardiac biomarkers were measured before and 8 and 24 hours after intervention. Patients with DM had significantly higher platelet reactivity before PCI compared to nondiabetics (214 ± 83 vs 193 ± 68 platelet reaction units, p = 0.02). HPR was more frequently observed in diabetics (36% vs 22%, p = 0.01) before PCI. Patients with DM had an increased incidence of periprocedural myocardial infarction (MI; 11% vs 4%, p = 0.04). When the entire population was divided by the presence or absence of DM and HPR, patients with DM and HPR presented the highest incidence of periprocedural MI (p for trend = 0.0008). HPR was an independent predictor of periprocedural MI (odds ratio 8.34, 95% confidence interval 2.60 to 26.76, p = 0.0003). In conclusion, patients with DM undergoing PCI have higher platelet reactivity at the time of PCI despite adequate clopidogrel pretreatment and subsequently worse periprocedural outcomes. Point-of-care platelet function testing may help to identify patients at higher risk of periprocedural MI.
The aim of this study was to prospectively evaluate the influence of diabetes mellitus (DM) on platelet reactivity measured by the VerifyNow P2Y12 assay and on periprocedural outcomes in patients receiving clopidogrel and undergoing percutaneous coronary intervention (PCI).
Methods
A total of 285 consecutive clopidogrel-treated patients undergoing elective PCI for stable angina or non–ST-elevation acute coronary syndromes were recruited. All patients received a 600-mg clopidogrel loading dose ≥6 hours before intervention or were pretreated with clopidogrel 75 mg/day for ≥5 days. Exclusion criteria were ST-elevation myocardial infarction (MI), upstream use of glycoprotein IIb/IIIa inhibitors, platelet count <70 × 10 9 /L, high bleeding risk, coronary artery bypass surgery in the previous 3 months, or severe renal failure (serum creatinine >2 mg/dl). After PCI, patients were treated with clopidogrel (75 mg/day) for ≥4 weeks after bare metal stent implantation and for 12 months after an acute coronary syndrome or drug-eluting stent implantation. All patients received aspirin before intervention and continued aspirin (100 mg/day) indefinitely. Procedural anticoagulation consisted of unfractionated heparin (100 U/kg) for all patients.
Platelet function analysis was performed in the cardiac catheterization laboratory immediately before PCI using the VerifyNow P2Y12 assay (Accumetrics, Inc., San Diego, California). This is a rapid cartridge-based turbidimetric whole-blood aggregation assay that measures the response to thienopyridines. Blood samples were collected in 2-ml Vacutainer tubes containing 3.2% sodium citrate. To avoid unwanted platelet activation the first 5 ml of blood was not used for platelet function testing. Technical details on the assay are described elsewhere. Platelet reactivity results are expressed in P2Y12 reaction units (PRUs). In general, the higher the PRU value, the lower the degree of platelet inhibition conferred by clopidogrel and vice versa.
Blood samples were also drawn from all patients before PCI and at 8 and 24 hours after intervention for measurement of creatine kinase-MB and troponin I levels. Additional samples were obtained to measure markers of myocardial necrosis if patients developed symptoms after PCI that were suggestive of myocardial ischemia. Measurements were performed using the Access 2 immunochemiluminometric assay (Beckman Coulter, Fullerton, California). Informed consent was obtained from all patients. No external source of funding supported this study.
Study end points were (1) evaluation of influence of DM on periprocedural platelet reactivity and (2) comparison of periprocedural outcomes in patients with and without DM according to platelet reactivity levels. DM was defined according to World Health Organization report criteria. High platelet reactivity (HPR) was defined as a PRU value ≥240 based on previous results from the Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-platelet Reactivity predicts Outcome (ARMYDA-PRO) study. Periprocedural MI was defined as a postprocedural increase in creatine kinase-MB >3 times the 99th percentile of the upper reference limit for patients with baseline negative myocardial necrosis markers, according to the Joint ESC/ACCF/AHA/WHF task force consensus statement on the redefinition of MI for clinical trials on coronary intervention. In patients with increased baseline levels of creatine kinase-MB, a subsequent increase ≥50% the baseline value fulfilled the criteria for periprocedural MI.
Continuous variables are expressed as mean ± SD, and categorical variables are reported as frequencies and percentages. Normality was tested by the Kolmogorov-Smirnov test. Continuous variables were compared by t test for normally distributed values; otherwise Mann-Whitney U test was used. Comparisons between categorical variables were made using Fisher’s exact test when the expected frequency was <5; otherwise chi-square test was applied. Odds ratios and 95% confidence intervals investigating the independent association of clinical and procedural variables with periprocedural MI were assessed by logistic regression. All variables listed in Tables 1 and 2 showing a significant univariate association with periprocedural MI (p <0.05) and HPR were entered in a multivariable logistic regression model. A p value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS 15.0 (SPSS, Inc., Chicago, Illinois).
| Variable | DM | p Value | |
|---|---|---|---|
| Yes (n = 104) | No (n = 181) | ||
| Age (years) | 67 ± 8 | 66 ± 9 | 0.33 |
| Men | 83 (80%) | 141 (78%) | 0.71 |
| Body mass index (kg/m 2 ) | 28.1 ± 6.1 | 26.8 ± 5.7 | 0.08 |
| Systemic hypertension ⁎ | 81 (78%) | 145 (80%) | 0.66 |
| Hypercholesterolemia † | 81 (78%) | 134 (74%) | 0.31 |
| Current smokers | 17 (16%) | 37 (20%) | 0.40 |
| Previous myocardial infarction | 30 (29%) | 58 (32%) | 0.57 |
| Previous coronary intervention | 37 (36%) | 73 (40%) | 0.43 |
| Previous bypass surgery | 8 (8%) | 8 (4%) | 0.25 |
| Non–ST-elevation acute coronary syndrome | 56 (54%) | 98 (54%) | 0.96 |
| Left ventricular ejection fraction (%) | 56 ± 8 | 55 ± 7 | 0.29 |
| Serum creatinine (mg/dl) | 1.11 ± 0.3 | 1.09 ± 0.2 | 0.55 |
| Baseline therapy | |||
| Insulin | 29 (28%) | — | — |
| Clopidogrel (before treatment) | 28 (27%) | 43 (24%) | 0.55 |
| Aspirin | 104 (100%) | 181 (100%) | 1 |
| Proton pump inhibitors | 31 (30%) | 55 (33%) | 0.92 |
| Statins | 93 (89%) | 156 (86%) | 0.43 |
| β blockers | 29 (28%) | 63 (35%) | 0.23 |
| Angiotensin-converting enzyme inhibitors | 87 (84%) | 138 (76%) | 0.14 |
⁎ Defined as systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg or current antihypertensive treatment.
† Defined as total cholesterol level >200 mg/dl or statin therapy.
| Variable | DM | p Value | |
|---|---|---|---|
| Yes (n = 104) | No (n = 181) | ||
| Coronary artery treated | 0.96 | ||
| Left anterior descending | 56 (54%) | 94 (52%) | |
| Left circumflex | 22 (21%) | 40 (22%) | |
| Right | 25 (24%) | 46 (25%) | |
| Saphenous vein graft | 1 (1%) | 1 (1%) | |
| Lesion type B2/C | 72 (69%) | 116 (64%) | 0.38 |
| Multivessel intervention | 15 (14%) | 29 (16%) | 0.72 |
| Type of intervention | 0.77 | ||
| Balloon only | 6 (6%) | 12 (7%) | |
| Stent | 98 (94%) | 169 (93%) | |
| Number of stents/patient | 1.3 ± 0.8 | 1.4 ± 0.9 | 0.33 |
| Stent diameter (mm) | 3.1 ± 0.9 | 3.0 ± 0.5 | 0.30 |
| Total stent length (mm) | 16 ± 10 | 14 ± 10 | 0.11 |
| Use of drug-eluting stents | 39 (38%) | 59 (33%) | 0.40 |
| Stent deployment pressure (atm) | 13 ± 6 | 14 ± 6 | 0.68 |
| Bailout use of glycoprotein IIb/IIIa inhibitors | 7 (7%) | 11 (6%) | 0.83 |
Results
One hundred four diabetic (36%) and 181 nondiabetic patients were enrolled. The main clinical and procedural features are presented in Tables 1 and 2 .
Patients with DM had significantly higher platelet reactivity before PCI compared to nondiabetics (214 ± 83 vs 193 ± 68 PRU, p = 0.02). HPR was more frequently observed in diabetics versus nondiabetics (36% vs 22%, p = 0.01; Figure 1 ) before PCI. This association remained significant after adjustment for factors potentially related to HPR (i.e., age, gender, body mass index, smoke, statin treatment, proton pump inhibitor treatment; odds ratio 1.895, 95% confidence interval 1.11 to 3.21, p = 0.02). There was no significant difference in the incidence of HPR in diabetic patients regardless of whether they were treated with insulin therapy or oral hypoglycemic drugs (p >0.10). In the quartile with the highest platelet reactivity before PCI, DM was more prevalent compared to the 3 lowest quartiles (47% vs 33%, p = 0.04).