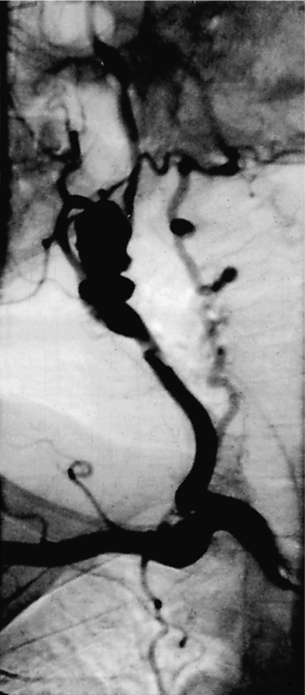
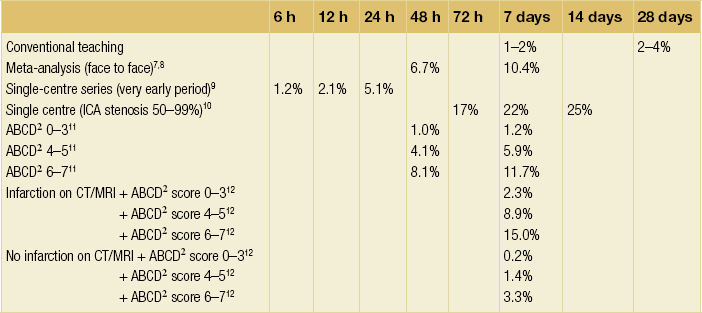
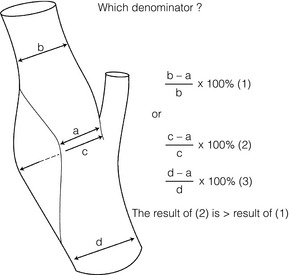
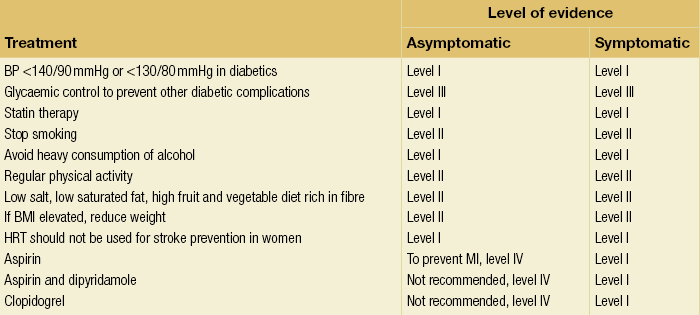
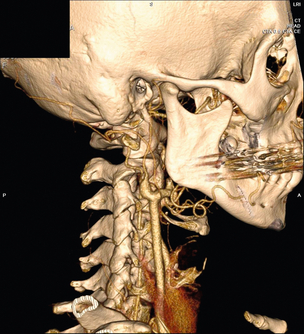
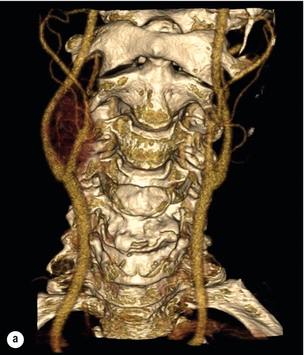
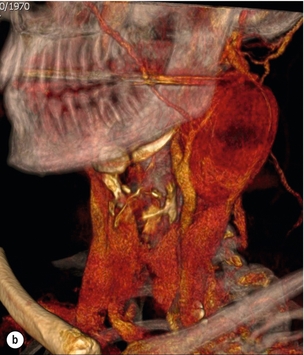
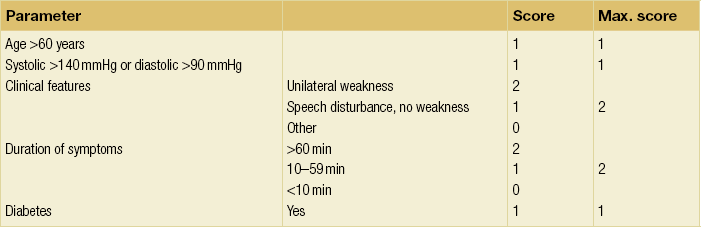
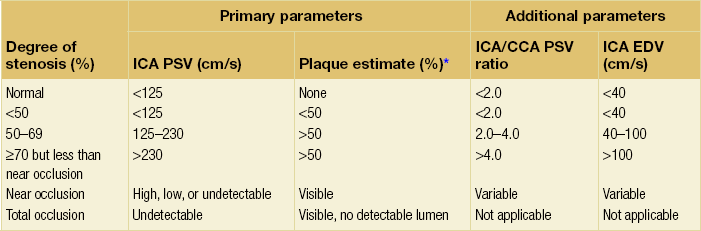
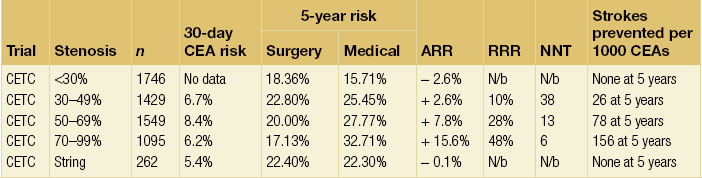
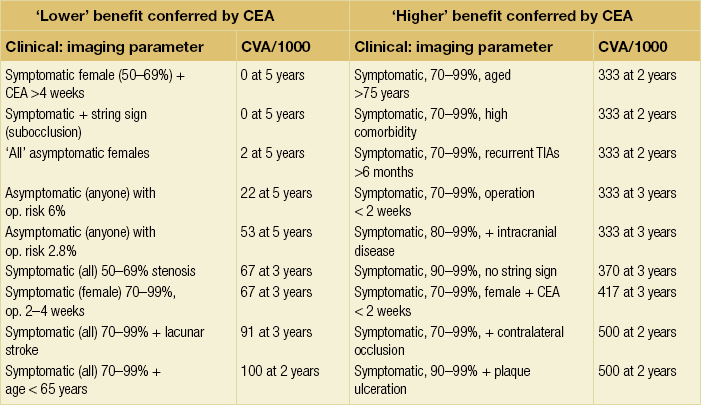
10 Stroke is the third commonest cause of death and the principal cause of neurological disability. It is defined as an acute loss of focal cerebral function with symptoms exceeding 24 hours (or leading to death), with no apparent cause other than that of a vascular origin. A transient ischaemic attack (TIA) has the same definition but lasts < 24 hours. In the UK, the annual incidence of stroke is 2 per 1000 and 125 000 patients will suffer their first stroke each year.1 Half of all strokes affect patients > 75 years of age. Stroke patients use 10% of inpatient beds and 5% of healthcare expenditure.2 Although mortality has diminished by about 20%, attributed to improved survival rather than a decline in incidence, the incidence of stroke could increase by 30% by 2033 because of the ageing population.3 About 36 000 patients will suffer a TIA each year, giving an annual UK incidence of 0.5 per 1000. TIA incidence increases with age from 0.9 per 1000 (55–64 years) to 2.6 per 1000 (75–84 years).4 About 80% of strokes are ischaemic, the remainder haemorrhagic (intracerebral/subarachnoid). Approximately 80% of ischaemic strokes affect the carotid territory. Risk factors include increasing age, smoking, hypertension, ischaemic heart disease, cardioemboli, previous TIA, diabetes, peripheral vascular disease, high plasma fibrinogen and hypercholesterolaemia. The principal causes of ischaemic, carotid territory stroke are: thromboembolism of the internal carotid artery (ICA) or middle cerebral artery (MCA) (50%); small-vessel intracranial disease (25%); cardiac embolism (15%); haematological disorders (5%); and non-atheromatous disease (5%).4 FMD is a rare disorder of unknown aetiology affecting medium-sized arteries, including the renal (60–75%) and carotid arteries (25–30%), in young to middle-aged women. The commonest presentation is hypertension. FMD is classified as: (i) intimal fibroplasia; (ii) medial dysplasia (medial fibroplasia, perimedial fibroplasia, medial hyperplasia); and (iii) adventitial (periarterial) fibroplasia. The commonest is medial fibroplasia (75–80% of cases), characterised by alternating stenotic webs and dilatation/aneurysm formation (Fig. 10.1). In up to 60%, FMD is bilateral. Patients with carotid FMD may be asymptomatic or symptomatic (TIA/stroke, dissection, false aneurysm). Although management tends to be conservative in asymptomatic individuals, surveillance is recommended. Once symptomatic, patients should be treated as for symptomatic atherosclerotic disease. Options include resection and interposition bypass, open graduated internal dilatation or (more commonly) percutaneous angioplasty. Dissection causes 2% of strokes, increasing to 20% in young adults (Fig. 10.2). One-fifth of trauma patients with an unexplained neurological deficit will have suffered a dissection and 25% will be bilateral. Dissection can be spontaneous (fibromuscular dysplasia), iatrogenic (rarely following angioplasty/stenting), be part of a central dissection (type A thoracic dissection) or follow blunt trauma (direct crushing, forced hyperextension or forced rotation) with crushing of the ICA between the mastoid process and the transverse process of C2. Figure 10.2 Three-dimensional CT angiogram showing typical features of an acute dissection of the right ICA. The dissection starts 2–3 cm above the true bifurcation and blood in the false lumen compresses the true lumen (the narrow channel extending up to the skull base). Patients suspected of having a dissection should undergo ultrasound and computed tomography (CT) or magnetic resonance angiography (MRA). This typically shows the dissection to start 2–3 cm beyond the origin of the ICA (Fig. 10.2). The distal limit is variable (occasionally as high as the petrous segment) with varying combinations of stenosis, dilatation, intimal flaps and occlusion in the intervening segment. The majority are managed conservatively. The aim is to reduce the risk of thrombosis and embolism. The carotid body is located within the adventitia of the posterior aspect of the carotid bifurcation and is responsible for monitoring blood gases/pH. A carotid body tumour (CBT) is derived from cells originating from the neural crest ectoderm (chemoreceptor cells), is typically located in the space between the ICA and external carotid arteries (ECA), and consists of nests of neoplastic epithelioid chief cells. As it enlarges, the bifurcation splays (Fig. 10.3a) and the patient becomes aware of a neck swelling. Other presentations include pain, invasion/compression causing hoarseness, cranial nerve palsies and Horner’s syndrome. CBTs rarely present with cerebral ischaemia, but can present as a hormonally mediated syndrome comprising flushing, dizziness, arrhythmias and hypertension. Figure 10.3 (a) Three-dimensional reconstruction of a CT angiogram of a highly vascular right carotid body tumour causing splaying of the bifurcation. In addition to providing information about the size and location of the tumour, this type of imaging is useful to exclude bilateral involvement (present in 5%). Note that the majority of the tumour circulation arises from branches of the ECA. (b) Three-dimensional CT angiogram of a probable glomus vagale tumour. Note that the tumour does not splay the bifurcation, but it pushes between the ECA and ICA from a posterior location higher up in the neck. Differential diagnoses include glomus vagale/ glomus jugulare tumours. The glomus vagale tumour arises from chemoreceptor cells within the vagus nerve and can be differentiated from a CBT because the bifurcation is not splayed. Instead, the tumour causes deviation of the ICA above the bifurcation (Fig. 10.3b). It is important to consider a glomus vagale tumour preoperatively as resection leads to swallowing problems (injury to motor fibres) and hoarseness (recurrent laryngeal nerve) and the patient has to be warned of this possibility. Asymptomatic cerebrovascular disease Four per cent of patients > 45 years will have a bruit, increasing to 12% in those > 60 years.6 One-third of patients with a 70–99% ICA stenosis will not have a bruit, increasing to 60% for those with 90–99% stenoses. Paradoxically, up to 30% of patients with an ICA occlusion will still have an audible bruit. The commonest reasons for a false-positive bruit are systolic cardiac murmurs, haemodynamic causes and bruits arising from the vertebrals or ECA. ‘Classical’ carotid territory symptoms include: (1) hemimotor/sensory signs, (2) transient monocular blindness (TMB) and (3) higher cortical dysfunction (Box 10.1). TMB usually develops over a few seconds and clears within a few minutes. Failure to resolve within 24 hours is analogous to a stroke. Conventional teaching has been that the risk of stroke after suffering a TIA/minor stroke is 1–2% at 7 days and 2–4% at 30 days. This, in conjunction with a reluctance to perform carotid surgery within the hyperacute period (because of concerns about increased procedural risks), has led to there being no real urgency regarding referral, investigation and management. However, there is now compelling evidence (Table 10.1) that the risk of stroke may be as high as 10% within 7 days of the index event,7,8 with almost half of these strokes occurring within the first 24 hours.9 The stroke risk may be as high as 20% at 7 days in TIA patients with a 50–99% carotid stenosis.10 There is good evidence that the early risk of stroke can be predicted from the ABCD2 score11 (see below). With increasing awareness that TIAs should be investigated and treated urgently, single-visit, rapid-access clinics have been established. In most UK centres, referrals are triaged using the ABCD2 scoring system (Table 10.2). The decision to refer should never be influenced by the presence/absence of a carotid bruit.12 The ‘ABCD2’ scoring system11 allocates a score based on five bedside parameters (A = age, B = blood pressure, C = clinical features, D = duration of symptoms, D = diabetes), with ‘7’ being the maximum). The early risk of stroke increases rapidly as the ABCD2 score increases (Table 10.1). Table 10.2 ABCD2 scoring system for predicting the 7-day risk of stroke after TIA Data derived from Johnston SC, Rothwell PM, Nguyen-Huynh N et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–92. A recent modification to the ABCD2 score has been the inclusion of whether or not there is CT/magnetic resonance imaging (MRI) evidence of infarction.13 In a prospective, multicentre series (4574 patients), the presence of CT/MRI infarction increased the early risk of stroke, irrespective of the ABCD2 score (Table 10.1). Specifically, patients with CT/MRI infarction and a low ABCD2 score had a similar early risk of stroke to patients with no evidence of infarction but a high ABCD2 score. Vertebrobasilar (VB) symptoms (Box 10.1) include bilateral blindness, problems with gait/stance, hemilateral/bilateral motor or sensory impairment (10% will have hemisensory/motor signs), dysarthria, homonymous hemianopia, nystagmus, dizziness, diplopia and vertigo (provided the latter three are not isolated). Duplex can only insonate the cervical portion of the extracranial carotid artery and is therefore relatively unreliable at excluding disease elsewhere. In diabetic patients, the incidence of tandem distal ICA stenoses varies between 14% and 21.3%, while 17–24% will have tandem intracranial disease.17 Any suspicion of additional lesions requires alternative imaging (e.g. MRA). The Society of Radiologists in Ultrasound Consensus Conference16 developed consensus criteria for diagnosing the severity of carotid disease based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET) measurement method (Table 10.3). Table 10.3 Society of Radiologists in Ultrasound Consensus Conference on ultrasound criteria for measuring carotid stenosis using the NASCET measurement method *Plaque estimate (diameter reduction) with grayscale and colour Doppler US. Reproduced from Grant EG, Benson CB, Moneta GL et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis – Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003; 229:340–6. With permission from the Radiological Society of North America. The identification of the ‘vulnerable plaque’, i.e. those more likely to cause thromboembolic complications, has proved difficult to achieve with ultrasound. The Gray–Weale classification18 classifies plaques according to whether they are echolucent (type 1), predominantly echolucent (type 2), predominantly echogenic (type 3) or echogenic (type 4). Unfortunately, correlation with histology and clinical risk is variable. An objective ultrasound parameter, the grey-scale median (GSM), has been shown to reliably differentiate between plaques associated with retinal and cerebrovascular symptomatology and asymptomatic status.19 There are, however, conflicting reports regarding the correlation between plaques considered to be vulnerable on the basis of a GSM ≤ 25 and increased procedural risk during CAS.20,21 In the modern era of high-quality non-invasive imaging there is no role for routine catheter angiography. Arch angiography is employed in some CAS centres as a means of assessing anatomic suitability for CAS and the status of the aortic arch/arch origins of the great vessels. It is associated with a much lower rate of stroke than selective angiography.22 There are three methods for measuring stenosis severity, each using the luminal diameter at the point of maximum stenosis as the numerator (Fig. 10.4). Stenoses measured using the ECST method generate higher grades than those using the NASCET method (Table 10.4). However, while the CCA method may be the most reproducible, most cerebrovascular centres now use the NASCET measurement method. Table 10.4 Correlation between ECST and NASCET measured carotid stenoses MRA uses flowing blood (time of flight) or gadolinium (contrast-enhanced MRA, CEMRA) as the contrast agent. CEMRA is not so dependent on vessel orientation and the field of view can be extended to include the arch of aorta and intracranial vessels (Fig. 10.5). Figure 10.5 CEMRA in the right anterior oblique orientation providing overview anatomical imaging, i.e. from the arch origin to the circle of Willis. There is an extremely tight stenosis (> 95%) at the right carotid bulb/proximal internal carotid artery. Although many studies comparing MRA with catheter angiography are methodologically flawed, a few are worthy of mention. One compared 71 bifurcations in 39 symptomatic patients with ultrasound, CEMRA and digital subtraction angiography (DSA).24 For the detection of ‘surgically appropriate’ lesions, CEMRA was found to have a sensitivity of 95% and a specificity of 79%, with 10% false-positive and 2.5% false-negative rates. Ultrasound had similar accuracy. However, if ultrasound and CEMRA were concordant (80% of cases), then all 70–99% stenoses were correctly identified and there were only 8.4% false-positive results. These ‘false positives’ were in the 60–65% category of stenoses. A second study (50 patients) found that CEMRA misclassified 24% of surgically amenable lesions (ultrasound misclassified 36%), but when CEMRA and ultrasound were concordant (48% of lesions) there was 100% sensitivity and only 17% of lesions were misclassified.25 Angina therapy should be optimised as the principal cause of late death is cardiac. Blood pressure (BP) should be maintained < 140/90 mmHg. Systematic reviews suggest that reducing diastolic BP by 5 mmHg lowers the relative risk of stroke by 35%, while the relative risk of myocardial infarction (MI) falls by 25%.29 However, evidence suggests that only 60% with known hypertension will receive treatment prior to suffering their first stroke and only half will have a documented diastolic BP <90 mmHg.30 The Heart Protection Study31 has provided level 1, grade A evidence for the role of statin therapy in patients with cerebrovascular disease. Antiplatelet therapy should be commenced in all patients unless contraindicated. There has been controversy over aspirin dosage during CEA. NASCET reported that patients receiving high-dose aspirin (650–1300 mg) had a lower perioperative risk than patients taking 0–325 mg aspirin daily.33 This was an unplanned analysis and a randomised trial of 2849 patients subsequently showed that the risks of stroke, MI and death within 30 and 90 days of CEA were significantly lower in patients receiving 80–325 mg as opposed to 650–1300 mg.34 This suggests greater evidence for prescribing low-dose aspirin. The early risk of stroke after suffering a TIA is higher than previously thought. This has led to a review of practice regarding the timing of surgery (see later), but also regarding the benefit of very early implementation of ‘best medical therapy’. The EXPRESS study evaluated early stroke risk in two cohorts of TIA patients. In the first cohort (2002–2004), patients were seen in a dedicated daily TIA clinic (appointment based, usual referral delays, etc.) with treatment recommendations being faxed to the referring doctor. The patient then contacted their doctor to obtain their prescription, but an average of 19 days elapsed before these medications were started. In the second cohort (2004–2007), there was a daily ‘walk-in’ service and statin, antiplatelet therapy was started in the outpatient clinic.35 The 90-day risk of stroke fell from 10.3% in the first cohort to 2.1% in the second. This reduction in risk was independent of age and gender, and rapid commencement of therapy was not associated with an increased risk of haemorrhagic stroke. Symptomatic carotid artery disease The Carotid Endarterectomy Trialists Collaboration (CETC) combined data from ECST, NASCET and the Veteran’s Affairs (VA) trials, having remeasured the pre-randomisation angiograms using the NASCET measurement method. This unique database36–38 includes 5-year outcomes in > 6000 patients (Table 10.6). Table 10.6 Carotid Endarterectomy Trialists Collaboration: 5-year risk of any stroke (including 30-day stroke/death) from the combined VA, ECST and NASCET trials Data derived from the CETC36–38 with all pre-randomisation angiograms remeasured using NASCET method. Following the publication of ECST/NASCET, there were concerns that the results may not be generalisable into clinical practice. For example, <0.5% of patients undergoing CEA in North America in 1988–9 were randomised into NASCET. At present, 94% of CEAs in the USA are performed in non-NASCET hospitals with a mortality significantly higher than observed in NASCET.39 The controversial issue regarding the relationship between hospital volume and outcome has been addressed in a systematic review of death/stroke after 936 436 CEAs.40 ECST/NASCET have published over 50 papers since 1991, most being secondary analyses that have increased knowledge about the role of CEA in patients with symptomatic cerebral vascular disease.41 These data should not be used to exclude patients from intervention, but rather to identify clinical and imaging predictors of increased risk of stroke on ‘best medical therapy’ (Box 10.2) and who derives the ‘least’ and ‘greatest’ benefit from CEA (Table 10.7). One of the most topical issues facing practitioners of both CEA and CAS is the effect of delay to intervention. Previously, there was no great impetus for expediting intervention other than recommending that CEA should be performed ‘as soon as reasonably possible’. Table 10.7 Predictors of relative benefit conferred by CEA CVA/1000, number of strokes prevented per 1000 patients treated. Derived from secondary analyses from ECST, NASCET, ACAS, ACST and CETC.
Extracranial cerebrovascular disease
Introduction
Aetiology and risk factors
Non-atheromatous carotid diseases
Carotid dissection
Carotid body tumour
Presentation of carotid disease
Symptomatic cerebrovascular disease
Vertebrobasilar
Investigation of carotid disease
Duplex ultrasound
Catheter angiography
NASCET (%)
ECST (%)
30
65
40
70
50
75
60
80
70
85
80
90
90
95
Magnetic resonance angiography
Management of cerebrovascular disease
Surgical management of carotid disease
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Extracranial cerebrovascular disease
Only gold members can continue reading. Log In or Register to continue