INTRODUCTION
History
The first human-human heart transplant was performed on December 3rd, 1967, in Cape Town, South Africa, by Dr. Christiaan Barnard. This operation was performed on a 53-year-old man named Louis Washkansky, who had ischemic cardiomyopathy. The first transplant in a child was only 3 days later, performed by Dr. Adrian Kantrowitz in Brooklyn, New York. The patient was a 17-day-old infant with Ebstein anomaly and functional tricuspid atresia. The patient died within hours of the transplant due to acidosis, thought to be due to respiratory causes. Due to poor outcomes of early transplantation in children and adults, pediatric heart transplantation was essentially discontinued, and was not pursued actively again until the early 1980s. Since that time, transplantation has become accepted as an option for end-stage heart failure in children.
Current Incidence and Outcomes
Approximately 350–400 heart transplants are performed in children under the age of 18 years annually in the United States (Fig. 28.1). Around 25%–30% of these transplants are performed in infants less than one year of age, while 30%–35% are performed in patients between the ages of 11 and 17 years. In infants, congenital heart disease (CHD) is the most common indication for transplantation (Fig. 28.2). While transplantation may be performed for a myriad of reasons in infants with CHD, hypoplastic left heart syndrome (HLHS) is the most common congenital indication. In adolescents, cardiomyopathies (dilated, restrictive, hypertrophic) are the predominant indication for transplantation.
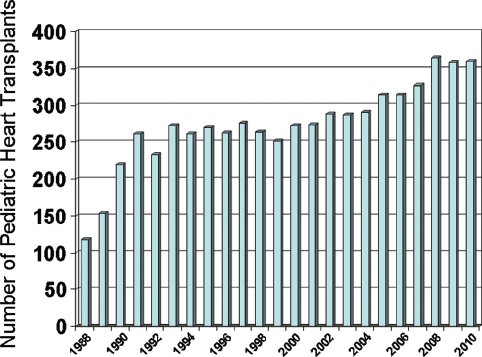
Figure 28.1. Number of heart transplants performed in children annually in the United States. (Data courtesy of UNOS).
Survival after pediatric heart transplantation is highly dependent on many factors. Pretransplant mechanical support, presence of congenital heart disease (i.e., non-cardiomyopathy), renal dysfunction, and hepatic dysfunction all portend worse outcomes after transplant. Posttransplant causes of mortality include acute allograft rejection, nonspecific graft failure, coronary artery vasculopathy, and malignancy. Survival rates after transplant according to International Society of Heart-Lung Transplant (ISHLT) data from after the year 2000 are 88%, 76%, and 62% at 1, 5, and 10 years, respectively.
Importance of Echocardiography
Echocardiography plays a critical role in the evaluation of the patient with end-stage heart failure, including diagnostic and specific functional assessments. Additionally, it is the current gold standard for assessment of potential donors. While routine assessment of the patient after transplantation requires several modalities working in concert, including interventional catheterization and laboratory studies, echocardiography clearly plays a major role in the clinical care of patients before, during, and after transplantation.
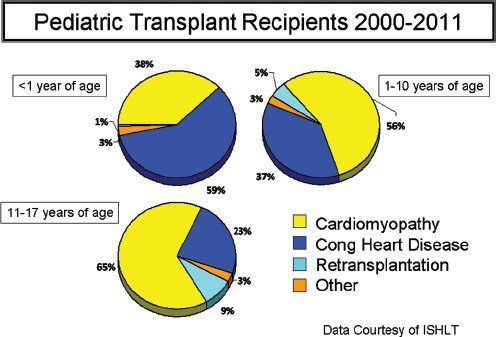
Figure 28.2.Indications for heart transplantation in children delineated by age group. (From Kirk R, Dipchand AI, Edwards LB, et al. The Registry of the International Society of Heart and Lung Transplantation: Fifteenth Pediatric Heart Transplantation Report – 2012. J Heart Lung Transplant. 2012;31(10):1065–1072.)
ECHO EVALUATION PRIOR TO TRANSPLANT
Evaluation of Potential Donors
The selection of appropriate donor hearts includes a thorough assessment of the donor’s past medical history, history of infectious or toxic exposures, current clinical and hemodynamic status (including invasive measurements of central venous pressure and pulmonary arterial pressure), and laboratory values. Perhaps the most useful tool, however, in the assessment of the potential donor heart is echocardiography. Echocardiography is useful because it is widely available at most hospitals, it is portable, it allows comprehensive evaluation of all chambers of the heart as well as the systemic and pulmonary veins and arteries, and it does not require the use of radiation or nephrotoxic contrast.
Brainstem death is associated with intensive sympathetic nervous system activity, and includes a large surge of catecholamines released into the circulation. This can cause a systemic inflammatory reaction, alter vascular resistances, and induce myocardial ischemia. Management of this sympathetic storm can be critical to maintaining the affected organs suitable for transplantation. Due in part to this sympathetic storm, left ventricular (LV) dysfunction commonly occurs in donors after neurologic injury and brainstem death. It may present with global dysfunction, or may present with regional wall motion abnormalities, even in the absence of coronary artery disease. The apex is most commonly spared, with the basal segments most affected.
In the absence of other risk factors for the donor heart, mild abnormalities of LV systolic function do not preclude the use of the heart. Outcomes of critically ill patients who received hearts with mild LV systolic functional abnormalities and mitral regurgitation have not differed from those who received donor hearts with normal systolic function. In particular, regional wall motion abnormalities seen in young donors typically do not persist after transplantation.
Although transthoracic echocardiography (TTE) usually is sufficient to assess the donor heart, in some cases, particularly in large adolescents, transesophageal echocardiography (TEE) may be necessary to delineate all anatomic structures and obtain a reliable assessment of cardiac function. Pharmacologic stress echocardiography has been proposed as an assessment of the marginal donor heart in adults. Preliminary data from studies in Europe using dipyridamole stress testing have shown that improvement in wall motion during stress in potential donor hearts is associated with normal function after transplant. While this methodology has not been well studied in pediatric patients, it carries promise for increasing the potential donor pool in the future.
In some cases, serial transthoracic echocardiography may be very useful, allowing for a repeat look at the potential donor heart after optimization of the donor’s metabolic and inflammatory conditions. Often, a heart that does not seem ideal may improve significantly on subsequent evaluation several hours later.
Evaluation of Potential Recipients
Echocardiography plays an important role in the evaluation and management of potential transplant recipients. It allows for assessment of chamber dimensions, valvular function, and systolic function of the ventricles, all of which correlate with risk of mortality while awaiting transplantation. In addition, echocardiography allows assessment of right-sided heart function and pressures. It is critical in the differentiation of constrictive pericardial disease (where the treatment is pericardiectomy) from restrictive cardiomyopathy (where the treatment is transplantation). Finally, echocardiography can be useful in the assessment and management of dyssynchrony.
Perhaps most importantly to the pediatric population, echocardiography plays an important role in identifying residual lesions in patients with congenital heart disease. These lesions may be contributing to the patient’s current “end-stage” heart failure symptoms, or are lesions that will require addressing at the time of transplantation. For instance, residual coarctation may be addressed at the time of transplantation in patients with a history of HLHS. Patients with heterotaxy, situs abnormalities, pulmonary venous abnormalities, interrupted inferior vena cava (IVC), or a left superior vena cava (SVC) may require modification of the surgical technique of transplantation, and are important to identify and plan for prior to the transplant.
ECHO EVALUATION DURING TRANSPLANT
Intraoperative TEE
TEE often will be performed in the operating room as the allograft is reperfused, to assess for any residual cardiac lesions, as well as to assess graft function. To adequately assess residual cardiac lesions and the graft anastomoses, an understanding of the specific surgical technique used is required. Traditionally, orthotopic heart transplantation (where one heart is removed and the other replaces it) was performed using the biatrial technique, where the surgeon anastomoses the allograft to retained cuffs of both left and right atrial tissue. With this technique, both atria will appear large on echocardiograms due to the presence of significant amounts of recipient and donor atrial tissue. Often, the suture lines will be visible in the mid-left and mid-right atrium. More recently, some surgeons perform transplantation using the bicaval technique. This involves leaving a cuff of left atrial tissue, leaving the pulmonary vein orifices intact, but removing the entirety of the right atrium by transecting the vena cavae. The allograft is then anastomosed to the left atrial cuff and to the IVC and SVC. Rarely, a heterotopic transplant may be performed, where the native heart is left in situ and the donor heart is placed next to the native heart. This allows for continued function of the native heart, and is used at some institutions in patients with elevated pulmonary vascular resistance, who would not otherwise be appropriate candidates for orthotopic heart transplantation.
One of the most important roles of echocardiography in the operating room is in the assessment of allograft function. LV function often is decreased after reperfusion; this may be due to prolonged ischemic time or a marginal donor heart. Transient mechanical support may be needed to allow the allograft time to recover function.
Right ventricular (RV) function is critical to assess in the operating room and in the first 24 hours after transplantation. Prior to transplant, many patients will have pulmonary hypertension secondary to elevated left-sided filling pressures. Often, the RV of the allograft will struggle after reperfusion to maintain sufficient pressures for lung perfusion. Inotropic support or nitric oxide administration may be necessary to support RV function. This is particularly problematic in patients with restrictive cardiomyopathy as their indication for transplant.
Rarely, hyperacute rejection may occur, and is typically evident immediately in the operating room (Fig. 28.3). This may present clinically to the surgeon as discoloration of the myocardium and poor hemodynamic function. On TEE, this presents as poor systolic function and increased wall thickness. Hyperacute rejection fortunately is rare, but is important to identify, as appropriate and immediate treatment is crucial.
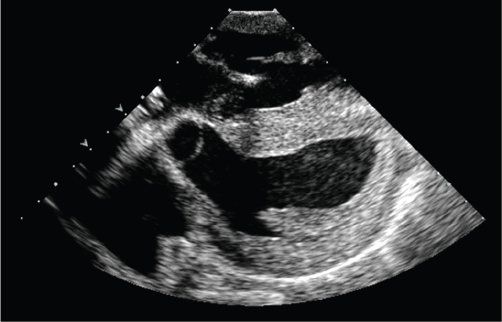
Figure 28.3. Epicardial echocardiogram performed on a 25-year-old female with hyperacute rejection, taken 4 days after transplant. The patient had presented with immediate systolic and diastolic dysfunction on reperfusion at the time of transplant, with progressive thickening of the myocardium over the first few days despite intensive antirejection treatment and ECMO rescue. Note the diffuse, infiltrative appearance of the myocardium. (See Video 28.1.)
Residual Cardiac Lesions
Significant anastomotic stenoses are rare in the modern era of pediatric heart transplantation. Most stenoses, when present, are mild, and most commonly include supravalvar aortic or supravalvar pulmonary stenosis. However, any patient in whom a nontraditional technique is required due to anatomic concerns (situs abnormalities, pulmonary venous abnormalities, recurrent coarctation, interrupted inferior vena cava, or left superior vena cava) needs careful attention by the echocardiographer. Patients with a history of a bidirectional cavopulmonary anastomosis are at particular risk of having stenosis of the SVC anastomosis.
ECHO EVALUATION AFTER TRANSPLANT
Acute Allograft Rejection
Acute allograft rejection is one of the leading causes of significant morbidity and mortality in heart transplant patients in the first several years following transplant. The gold standard for diagnosis of rejection is pathologic examination of myocardial tissue, obtained from an endomyocardial biopsy (EMB). However, EMB is invasive, and carries risks, including tricuspid valve damage (leaflet perforation, disruption of chordae tendinae, injury to papillary muscles, all of which result in tricuspid regurgitation), myocardial perforation, arrhythmias, risks of repeated jugular and/or femoral venous access, and the standard risks of anesthesia.
Because of these risks, investigators have long sought to identify specific echocardiographic measures with which to reliably diagnose (or exclude) allograft rejection. With some notable exceptions, the majority of studies assessing echocardiography in pediatric heart transplant recipients have not demonstrated that echocardiography can reliably replace the EMB as the gold standard. However, there clearly is a role for echocardiography in the assessment of potential rejection, particularly in young children for whom repeated EMB is cumbersome, and in the asymptomatic patient with significant rejection.
Common Findings of Rejection
Pathologically, acute cellular rejection is characterized by lymphocytic infiltration, myocardial inflammation and edema, and, in advanced stages, myocardial necrosis. This disruption of myocyte function translates on the echocardiogram to myocardial thickening, pericardial effusion, systolic and diastolic dysfunction, and new valvar regurgitation, particularly of the atrioventricular valves. In many cases, this is very clear on echocardiography, particularly with severe episodes of rejection.
Interestingly, patients with acute rejection often can present in very different ways on echocardiography. For some patients, the first sign of rejection on echocardiography might be a new pericardial effusion, or markedly increased wall thicknesses (Fig. 28.4). For others, new-onset atrioventricular valve regurgitation and/or systolic dysfunction may be the first sign (Fig. 28.5). Diastolic dysfunction may be seen in some patients in the absence of other findings. Relaxation abnormalities may be evident to the echocardiographer on two-dimensional imaging of the ventricles, or it may become evident on standard Doppler diastolic assessment including mitral inflow velocities and tissue Doppler imaging (TDI) (Fig. 28.6).
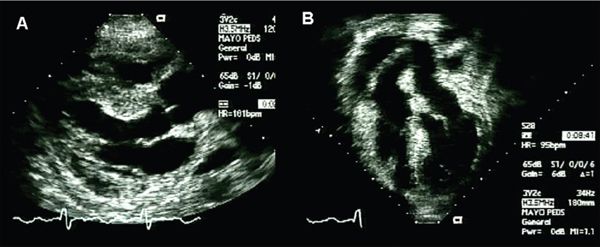
Figure 28.4. Routine transthoracic echocardiogram performed in a 17-year-old female two months after transplant, including parasternal long-axis (A) and apical (B) images. The patient was asymptomatic at the time of the echocardiogram. Note the diffuse thickening and “edematous” appearance of the myocardial walls, and new posterior pericardial effusion. Of note, this patient had normal systolic function and normal AV-valve function. (See Video 28.2.)
Importantly, increased LV mass and wall thickness may occur in patients with rejection, but this is difficult to discern from a common increase in LV mass that occurs after transplantation. In adult transplant recipients, the mean LV mass is around 35% greater than in age-matched controls. While not entirely elucidated, there are several factors that are thought to contribute to this increased LV mass, including transient myocardial injury/edema, hypertension, myocardial denervation, and the immunosuppressive regimen being used (particularly if steroids are used). The hypertrophy can be visualized easily using echocardiography. It may occur in a concentric pattern, or may occur primarily in the ventricular septum (Fig. 28.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


