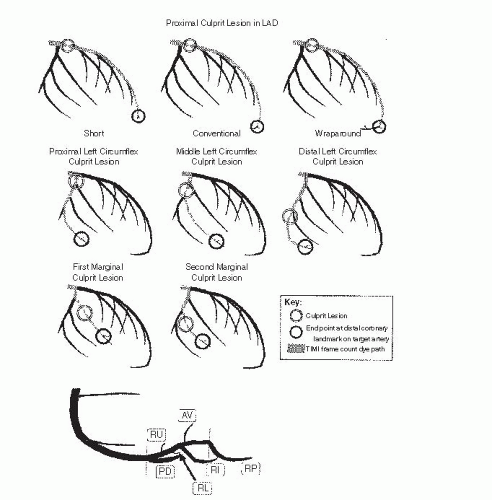
Measurement of myocardial metabolism may be performed noninvasively (e.g., positron emission tomography scanning) or invasively by transmyocardial sampling techniques that involve acquisition of simultaneous arterial and coronary venous (e.g., coronary sinus) blood. Specialized blood products commonly used in the determination of changes in myocardial metabolism include serum pyruvate, lactate, oxygen, and other metabolic or hematologic blood components. Transmyocardial extraction of pharmaceutical agents after systemic or intracoronary delivery can also be determined by transmyocardial blood sampling for the measurement of arterial-venous concentration difference, along with measurement of blood flow per unit time.
In studies involving ischemic myocardial metabolism, the most commonly measured products are lactate and oxygen. Specialized chilled collection tubes containing an agent (perchloric acid) to stop red cell metabolism and prevent clotting are used for chemical assays to measure small differences in normal lactate levels across the myocardium. Clinical laboratory tests calibrated for the high lactate levels in lactic acidosis are unsuitable for the measurement of the small transmyocardial differences. Myocardial catecholamines (norepinephrine, epinephrine) and other vasoactive mediator products, such as prostaglandins, can be measured if sample tubes are placed immediately in ice to prevent platelet activation after blood withdrawal through a long narrow catheter lumen. Large-bore (≥6F) heparin-coated catheters may be required to assess platelet products. Measurement of myocardial metabolism requires preparation of the sampling tubes and other collection materials using advanced techniques.
Regulation of Coronary Blood Flow and Resistance
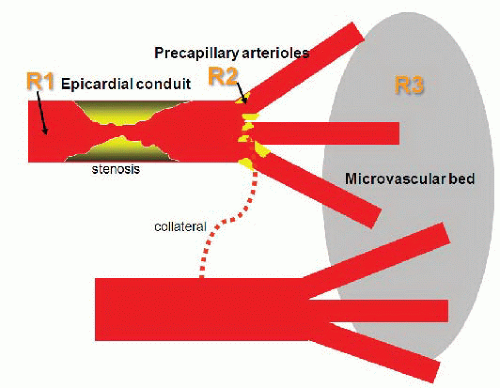
Coronary arterial resistance (
R, pressure ÷ flow) is the summed up resistances of the epicardial coronary conduit (
R1), and the precapillary arteriolar (
R2) and intramyocar-dial capillary (
R3) resistance circuits
8 (
Figure 24.1). Normal epicardial coronary arteries in humans typically taper from the base of the heart with diameters of typically 5 to 6 mm to the apex where the vessel diameter is typically down to 0.3 mm. The epicardial vessels do not offer appreciable resistance to blood flow (
R1) in their normal state. Even at the highest level of blood flow, there is no detectable resistance as would be manifest as a pressure drop along the length of human epicardial arteries,
9 making even large epicardial vessel resistance (
R1) trivial until atherosclerotic obstructions develop. Most of the epicardial vessel wall consists of a muscular media that responds to changes in aortic pressure and modulates coronary tone in response to flow-mediated endothelium-dependent vasodilators, circulating vasoactive
substances, and neurohumoral stimuli. Large-conduit arteries are unaffected by myocardial metabolites because of their extramural location, but can produce episodic increases in resistance during severe focal or diffuse contraction (vasospasm) in the absence of atherosclerosis. One exception is myocardial bridging, in which intramyocardial vessel segments may offer increased resistance during systole owing to mechanical compression of the bridged segment during ventricular contraction.
Changes in epicardial and arteriolar coronary resistances in response to physiologic or pharmacologic stimuli can be considered either primary or secondary vasomotor events. Primary vasodilation signifies an alteration in myocardial vessel tone and perfusion with no preceding change in myocardial oxygen demand. Secondary vasodilation refers to changes in vessel tone and blood flow that occur in response to alterations in myocardial oxygen consumption.
10Precapillary arterioles are resistive vessels (
R2) connecting epicardial arteries to myocardial capillaries and
are the principal controllers of coronary blood flow.
8 Precapillary arterioles (100 to 500 µm in size) contribute approximately 25% to 35% of total coronary resistance. The prearteriolar resistance function autoregulates the driving pressure at the origin of the precapillary arterioles within a finite pressure range. This regulatory function is also influenced by myogenic and flow-dependent vasodilatation related to shear stress.
The microcirculatory resistance (R3) circuit consists of a dense network of about 4,000 capillaries per square millimeter, which ensures that each myocyte is adjacent to a capillary. Capillaries are not uniformly patent because precapillary sphincters regulate flow according to oxygen demand. Several conditions, such as LV hypertrophy, myocardial ischemia, or diabetes, can increase the microcirculatory resistance (R3) and blunt the normal maximal increases in coronary flow. Increased R3 resistance may also be associated with elevated resting blood flow above that expected for the existing myocardial oxygen demand, resulting in reduced coronary flow reserve (i.e., the hyperemic/basal flow ratio).
As in any vascular bed, blood flow to the myocardium depends on the coronary artery driving pressure and the resistance produced by the serial vascular components. Coronary vascular resistance, in turn, is regulated by several interrelated control mechanisms that include myocardial metabolism (metabolic control), endothelial (and other humoral) control, autoregulation, myogenic control, extravascular compressive forces, and neural control. These control mechanisms may be impaired in diseased states, thereby contributing to the development of myocardial ischemia (
Tables 24.3 and
24.4).
Coronary vasodilatory reserve (CVR) is the ability of the coronary vascular bed to increase flow from a basal level to a maximal (or near maximal) hyperemic level in response to a mechanical or pharmacologic stimulus. Such
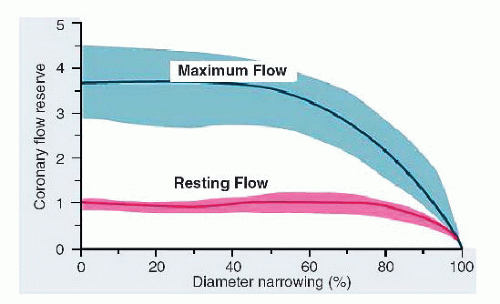
stimuli include the reactive hyperemia that follows transient coronary occlusion, exercise, and the administration of various pharmacologic agents. Coronary flow reserve is expressed as the ratio of maximal hyperemic flow to resting coronary flow—a ratio that averages from 4 to 7 in experimental animals and from 2 to 5 in man.
11,
12 In experimental animal studies, increasing conduit stenosis (
R1) produces a predictable decline in coronary flow reserve, beginning at about a 60% artery diameter narrowing. At diameter stenoses of >80% to 90%, all available coronary reserve has been exhausted and resting flow begins to decline
13,
14,
15 (
Figure 24.2). (Factors responsible for reduced CVR in the absence of epicardial stenosis are listed in
Table 24.5.) This relationship between increasing stenosis severity and reduced available flow reserve has been used in assessing the effective physiologic severity of any given coronary lesion and forms the basis of many noninvasive test modalities for ischemia. In clinical practice, however, for an individual patient, this relationship is unpredictable given the complex three-dimensional anatomy, imprecise correlation between angiographic estimate of diameter reduction owing to stenosis and true lumen cross-sectional area, and unknown status of microcirculation.
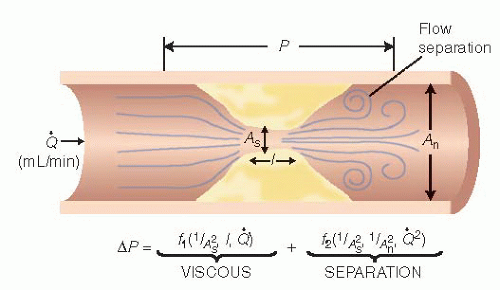
Furthermore, the influence of a stenosis on coronary blood flow is principally related to the morphologic features
16 of the stenosis, with resistance to flow changing exponentially with lumen cross-sectional area (the most commonly used measure of severity) and linearly with lesion length (
Figure 24.3). Additional factors contributing to stenosis resistance include the shape of the entrance and exit orifices, vessel stiffness, distensibility of the diseased segment (permitting active or passive vasomotion), and the variable lumen
obstruction that may be superimposed by platelet aggregation and thrombosis compromising lumen area, a process active in acute coronary syndromes (
ACSs).
16As blood traverses a diseased arterial segment, turbulence, friction, and separation of laminar flow causes energy loss resulting in a pressure gradient (ΔP) across the stenosis. Using a simplified Bernoulli formula for fluid dynamics, pressure loss across a stenosis can be estimated from blood flow as follows:
where Δ
P is the pressure drop across a stenosis in millimeters of mercury (mmHg),
Q is the flow across the stenosis in milliliters per second, and
dsten is the minimal diameter of the stenosis lumen in millimeters. In
Eq. (24.1), the first term (
f) accounts for energy losses owing to viscous friction between the laminar layers of fluid and the second term (
s) reflects energy loss when normal arterial flow is transformed first to high-velocity flow in the stenosis and then to the turbulent nonlaminar distal flow eddies at the exit from the stenosis (inertia and expansion).
Where As = stenotic segment cross-sectional area, p = blood density, µ = blood viscosity, L = stenosis length, and An = normal artery cross-sectional area.
It is important to note that the separation energy loss term (
s) increases with the square of the flow while viscous energy loss (
f) becomes negligible. Thus, increases in coronary blood flow increase the associated pressure gradient in an exponential manner. Despite augmentation of coronary blood flow, the increasing pressure loss across the stenotic segment reduces myocardial perfusion pressure and lowers the threshold for myocardial ischemia relative to demand.
17From
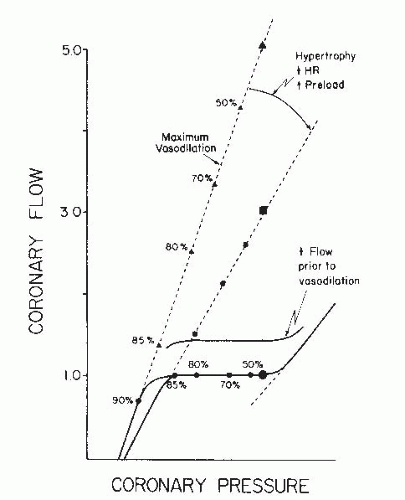
Eq. (24.2), the trans-stenotic pressure drop is inversely proportional to the fourth power of the lumen radius. As a consequence, in a severe stenosis, relatively small change in luminal diameter (such as caused by active or passive vasomotion or transient obstruction by thrombus) can produce marked hemodynamic effects. For example, when the diameter stenosis increases from 80% to 90%, the resistance of a stenosis rises nearly threefold. For most stenoses, the length of the narrowing has only a modest effect on its physiologic significance. However, in very long narrowed segments, signi ficant turbulence occurs along the walls of the stenotic segment and energy is dissipated as heat when eddies form and impact on the vessel wall. In addition, a preserved arc of vascular smooth muscle in some diseased arteries may be compliant and subject to dynamic changes that can alter luminal caliber and stenosis resistance. Dynamic changes in stenosis severity and resistance can also occur passively in response to changes in intraluminal distending pressure or selective dilation of distal resistance vessels. Thus for a given stenosis, there is a family of pressure-flow relationships reflecting altered stenosis diameter and variable distending pressure (
Figure 24.4).

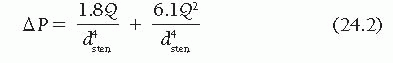
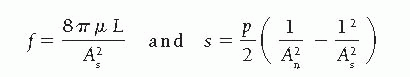


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access