Epidemiology of Lung Cancer
THE EPIDEMIOLOGY OF LUNG CANCER
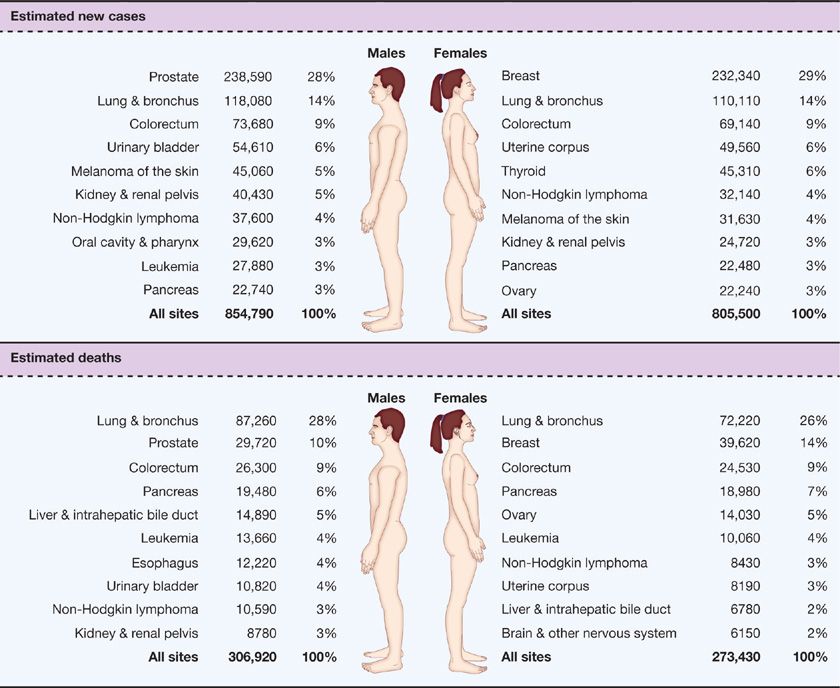
Lung cancer is the leading cause of cancer death in the United States and worldwide. Siegel et al.1 estimated a total of 246,210 new lung cancer cases and 163,890 deaths from lung cancer in the United States in 2013.1,2 These statistics reflect data ending in 2009, and likely underestimate the current lung cancer burden. In the United States, more Americans die of lung cancer every year than from prostate, breast, and colon cancer combined.1 Cancer of the lung and bronchus ranked second in cancer incidence in both sexes, with an estimated 118,080 new cases in males (14% of all new cancers) and 110,110 in females (14% of all new cancers).1 The age-adjusted incidence rate of lung cancer was 62 per 100,000 men and women per year in the United States, with the incidence rate much higher in men than in women (75.2 vs. 52.3 per 100,000).3 Lung cancer ranked first in both sexes in the number of estimated deaths yearly1 (87,260 or 28% of all cancer deaths for males and 72,220 or 26% of all cancer deaths for females) (Fig. 109-1). The current 5-year survival rate in the United States for lung cancer is 17%; while this rate has actually increased over the past few decades, it lags behind survival advances in other common malignancies.1
Figure 109-1 Ten leading cancer types for the estimated new cancer cases and deaths categorized by sex. (Reproduced with permission from Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.)
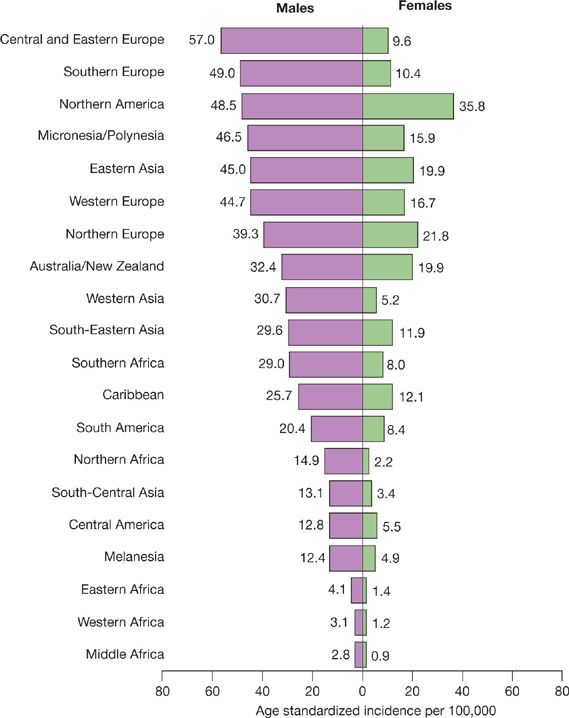
Globally, lung cancer has been the most common cancer since 1985, both in terms of incidence and mortality rate. Worldwide, lung cancer is the largest contributor to new cancer diagnoses (1,350,000 new cases; 12.4% of total new cancer cases) and to death from cancer (1,180,000 deaths; 17.6% of total cancer deaths).2 Worldwide, it was also the most commonly diagnosed cancer and the leading cause of cancer death in males in 2008.2 For females, lung cancer was the fourth most commonly diagnosed cancer and the second leading cause of cancer death. Lung cancer incidence and mortality rates are highest in the United States and the developed countries, and relatively lower in Central America and most of Africa (Fig. 109-2). However, there has been a large relative increase in the numbers of cases of lung cancer in developing countries. Almost half (49.9%) of the cases now occur in developing countries, whereas in 1980, 69% of cases were in developed countries. The estimated numbers of lung cancer cases worldwide has increased by 51% since 1985 (a 44% increase in men and a 76% increase in women).2 The World Health Organization estimates that lung cancer deaths worldwide will continue to rise, largely as a result of an increase in global tobacco use, especially in Asia.2
Figure 109-2 Age-standardized lung cancer incidence and mortality rates by sex and world area. Lung cancer incidence by sex and world area. Rates are standardized to the world standard population, in 2008. (Adapted with permission from Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.)
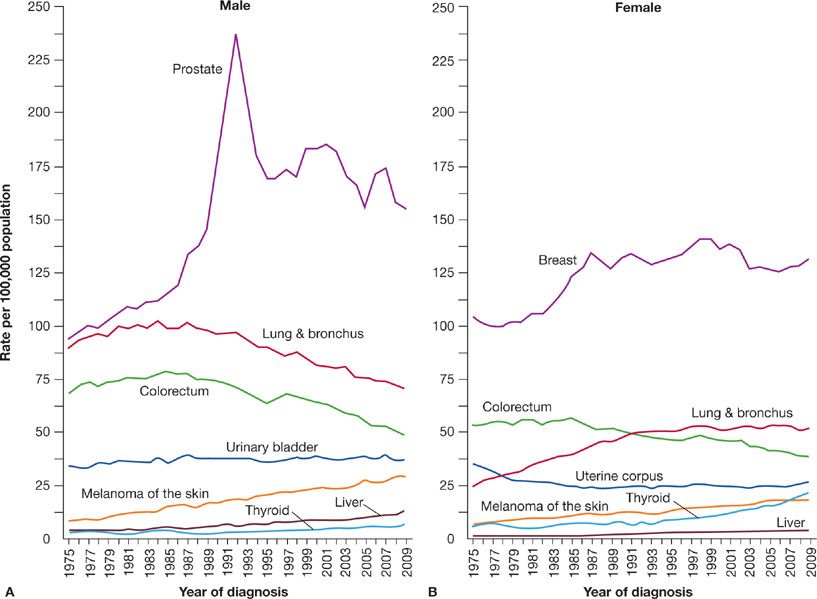
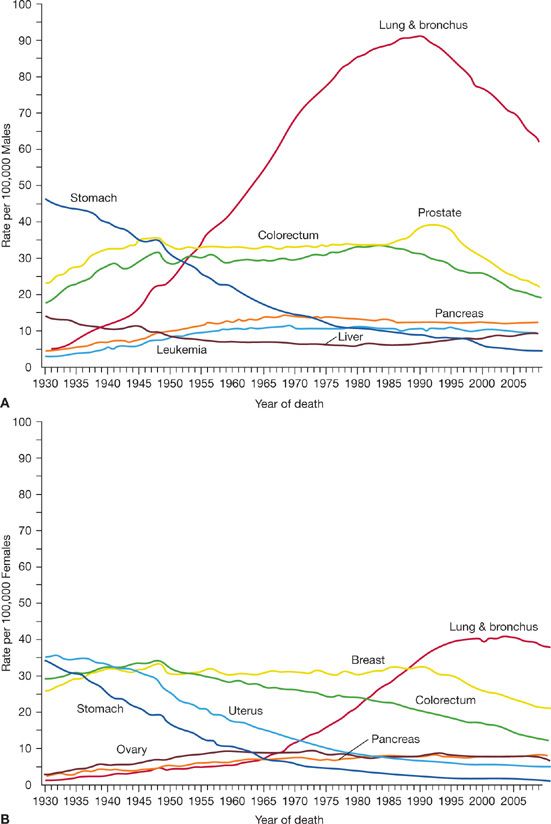
Lung cancer incidence in males in the United States has been decreasing since the early 1980s (Fig. 109-3). The incidence and mortality rates for lung cancer tend to mirror one another because most patients diagnosed with lung cancer eventually die of the disease. Siegel et al.4 noted decreases in death rates from lung cancer in men by 2.0% per year from 1994 to 2006 (Fig. 109-4). In women, lung cancer death rates instead increased by 0.3% per year from 1995 to 2005; however, more recent data from 2003 to 2006 show a decline of 0.9% per year (Fig. 109-4). Because of the change in lung cancer incidence in women, recent figures show that lung cancer death rates have decreased in women for the first time, more than a decade after the beginning of the decreasing trend in men.5 The difference between the sexes in the onset of decline in lung cancer may be attributable to the fact that cigarette smoking in women peaked two decades later than in men. Lung cancer mortality rates thus appear to be reaching a plateau for women, which is an encouraging change from the steep rise in the 1970s.
Figure 109-3 Trends in incidence rates for cancer of the lung and bronchus among (A) males and (B) females in the United States, 1975–2009. (Reproduced with permission from Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.)
Figure 109-4 Death rates trend for selected cancers including cancer of the lung and bronchus in the United States. Rates are age adjusted to the 2000 US standard population with data from 1930 to 2009 for (A) males and (B) females. (Reproduced with permission from Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.)
The Surveillance, Epidemiology, and End Results (SEER) data from 2004 to 2008 report the median age at diagnosis for cancer of the lung and bronchus to be 71 years. No cases were diagnosed in patients younger than 20 years.3 Approximately 0.2% of cases were diagnosed in patients between age 20 and 34 years; 1.5% between 35 and 44 years; 8.8% between 45 and 54 years; 20.9% between 55 and 64 years; 31.1% between 65 and 74 years; 29% between 75 and 84 years; and 8.3% at 85 years and older.
There are two broad categories of lung cancer: small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC). SCLC, which is a highly malignant tumor derived from cells exhibiting neuroendocrine characteristics, accounts for 15% of lung cancer cases. NSCLC, which accounts for the remaining 85% of cases, is further divided into three major pathologic subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Adenocarcinoma by itself accounts for 38.5% of all lung cancer cases, with squamous cell carcinoma accounting for 20%, and large cell carcinoma accounting for 2.9%.3,6 In the past several decades, the incidence of adenocarcinoma has increased greatly, and it has replaced squamous cell carcinoma as the most prevalent type of NSCLC.
Despite the availability of new diagnostic and genetic technologies, advancements in surgical techniques, and the development of new biologic treatments, the overall 5-year survival rate for lung cancer in the United States remains at a dismal 17%.7 The 5-year survival rate in Europe, China, and developing countries is estimated at only 8.9%.2 Lung cancer stage is often advanced at the time of diagnosis; 30% to 40% of cases of NSCLC and 60% of SCLC are stage IV at presentation. Patients with stage I disease at diagnosis have a 5-year survival rate of approximately 50%. In stark contrast, patients with advanced stage disease and distant metastasis at diagnosis have a dismal 5-year survival rate of 1% to 2%, which argues strongly for the need for better screening methods to detect early stage cancers.8,9
ETIOLOGY OF LUNG CANCER—TOBACCO SMOKING AND LUNG CANCER
Tobacco smoking is the most important modifiable risk factor for lung cancer. It has been estimated that up to 20% of all cancer deaths worldwide could be prevented by the elimination of tobacco smoking.10 More than 80% of lung cancers develop in smokers, and one in nine smokers develops lung cancer.11 The cumulative lung cancer risk among lifelong heavy smokers can be as high as 30% compared with a lifetime risk of less than 1% in nonsmokers.12,13 Smoking cessation, especially at a younger age, is associated with many health benefits including lowering the risk of lung cancer; cessation before the age of 40 years reduces the risk of death associated with continued smoking by about 90%.14 Lung cancer risk is proportional to the magnitude of cigarette consumption, as factors such as the number of packs per day smoking, the age of onset of smoking, the degree of inhalation, the tar and nicotine content of cigarettes, and use of unfiltered cigarettes are important.15,16 Individual susceptibility, which is a function of environmental factors and genetic predisposition, is a factor in carcinogenesis.
In 1912, Adler in an extensive review of autopsy reports from hospitals in the United States and western Europe identified 374 cases of primary lung cancer, representing <0.5% of all cancer cases.17 He concluded at the time that “primary malignant neoplasms of the lung are among the rarest forms of disease.” In 1920, lung cancer constituted only 1% of all malignancies in the United States, but over the next several decades, the incidence of lung cancer increased disproportionately to the incidence of all other cancers.18 The first report linking cigarette smoking with an increased risk of premature death was in 1938 when Pearl showed that cigarette smoking had a dose-related adverse effect on longevity.19 The finding that tar (the total particulate matter in cigarette smoke after water and nicotine are removed) applied to the skin of animals produced skin cancer raised concern that inhalation of tar products could be an important etiologic factor in lung cancer. Subsequent studies in patients and experimental animals demonstrated that tar from the burning of tobacco was carcinogenic.20 Ochsner and DeBakey21 stated in their 1941 review of lung carcinoma that “it is our definite conviction that the increase in the incidence of pulmonary carcinoma is due largely to the increase in smoking.”
In 1950, two large landmark epidemiologic studies established the causal role of tobacco smoking in bronchogenic carcinoma.22,23 In the United Kingdom, Doll and Hill described an association between carcinoma of the lung and cigarette smoking, and the effect of the number of cigarettes smoked on the development of lung cancer.19,22,24 In the United States, Wynder and Graham examined 605 cases of lung cancer in men and found that 96.5% of lung cancers were in men who were moderate to heavy smokers for many years.19 They concluded that (1) the excessive and prolonged use of tobacco was an important etiologic factor in lung cancer; (2) lung cancer in nonsmokers was rare; and (3) the onset of carcinoma could occur 10 years or more after the cessation of smoking.
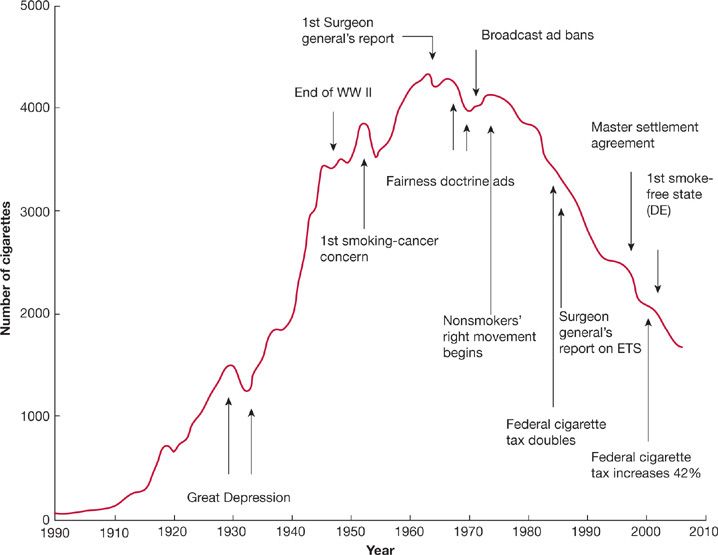
In 1964, the United States Surgeon General issued a landmark report on smoking and its effects on health that included a number of key observations and conclusions. First, cigarette smoking was associated with a 70% increase in the age-specific death rates of men and a lesser increase in the death rates of women. Second, cigarette smoking was causally related to lung cancer in men. The magnitude of the effect of cigarette smoking far outweighed all other factors leading to lung cancer. The risk for lung cancer increased with the duration of smoking and the number of cigarettes smoked per day. The report estimated that the average male smoker had an approximately 10-fold risk for lung cancer, whereas heavy smokers had at least a 20-fold risk. At that time, the relative risk for lung cancer death among smokers was five times as high for men than for women; however, these differences between men and women are no longer as evident.25 Third, cigarette smoking was believed to be much more important than occupational exposures in the causation of lung cancer in the general population. Fourth, cigarette smoking was the most important cause of chronic bronchitis in the United States. Finally, male cigarette smokers had a higher death rate from coronary artery disease than male nonsmokers. The report concluded that: “Cigarette smoking is a health hazard of sufficient importance in the United States to warrant appropriate remedial action.” Despite efforts to curb tobacco smoking, there are approximately 1.1 billion smokers worldwide, and if the current trends continue, that number will increase to 1.9 billion by the year of 2025.26 Wynder and Graham estimated that the average American adult smoked fewer than 100 cigarettes per year in 1900.27 Fifty years later, the number smoked had risen to approximately 3500 cigarettes per person per year and reached a maximum of approximately 4400 cigarettes per person per year in the mid-1960s (Fig. 109-5).28 Since the 1964 publication of the Surgeon General’s first report on the health consequences of smoking, yearly per capita consumption of cigarettes has been declining in the United States (Fig. 109-5).28 As of 2008, it is estimated that 20.6% of American adults over age 18 years old (46.0 million) are habitual smokers, an improvement from 24.1% a decade previously.29,30 Of these, 79.8% (36.7 million) smoke every day and 20.2% (9.3 million) smoke some days. Unfortunately, the rate of decline in smoking prevalence has slowed recently, with 19.5% of American adults habitually smoking in 2009.31 More than 80% of adult smokers begin before the age of 18 years. In 2009, one in five American high school students reported smoking cigarettes in the preceding 30 days.32 Several factors identify populations more likely to smoke. Prevalence is higher in men (23.5%) than in women (17.9%). Socioeconomic influences contribute. Among persons below the federal poverty level, the prevalence of smoking is 31.1%. In the population of adults older than 25 years, the prevalence of smoking among persons with educational status less than a high school diploma is 28.5% compared with 5.6% among persons with a graduate degree.30 There are also regional differences in the United States, with lowest smoking prevalence in western states (16.4%) compared to southern (21.8%) and midwest states (23.1%).29
Figure 109-5 The adult per capita cigarette consumption in the United States, 1900 to 2006, with historical highlights. (Adapted with permission from Warner KE, Mendez D. Tobacco control policy in developed countries: yesterday, today, and tomorrow. Nicotine Tob Res. 2010;12(9):876–887.)
Cigarette smoke is a complex aerosol composed of more than 4000 gaseous and particulate compounds. Mainstream smoke is produced by inhalation of air through the cigarette and is the primary source of smoke exposure for the smoker. Sidestream smoke is produced from smoldering of the cigarette between puffs and is the major source of environmental tobacco smoke (ETS). The primary determinant of tobacco addiction is nicotine; exposure to tar appears to be a major component of lung cancer risk. The nicotine and tar composition of mainstream smoke can vary greatly depending on the intensity of inhalation by the smoker. Although the use of filter tips decreases the amount of nicotine and tar in mainstream smoke, the effect of filter tips also varies in relation to differences in the compression of the filter tips by lips or fingers and the depth of inhalation of the smoker.
The primary factor determining intensity of cigarette use is the nicotine dependence of the smoker, and although cigarettes now contain less nicotine and tar than in the past, smokers tend to take more puffs per minute and inhale more deeply to satisfy their nicotine need. Low-yield filtered cigarettes have been hypothesized to contribute to the increase in the incidence of adenocarcinoma of the lung.33 The nicotine-addicted smoker smokes low-yield cigarettes far more intensively than nonfiltered higher-yield cigarettes. With deeper inhalation, higher-order bronchi in the peripheral lung, which lack protective epithelium, are exposed to carcinogen-containing smoke, rather than simply the major bronchi.
The International Agency for Research on Cancer (IARC) has identified at least 50 carcinogens in tobacco smoke.34,35 The agents that appear to be of particular concern in lung carcinoma are the tobacco-specific N-nitrosamines (TSNAs) formed by nitrosation of nicotine during tobacco processing and during smoking. Eight TSNAs have been described, including 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), which is known to be an important inducer of lung cancer. Mainstream smoke contains other potential carcinogens, including polycyclic aromatic hydrocarbons (PAHs), aromatic amines, and other organic and inorganic compounds such as benzene, vinyl chloride, arsenic, and chromium. The PAHs and TSNAs require metabolic activation to become carcinogenic. Metabolic detoxification of these compounds can also occur, and the balance between activation and detoxification likely affects individual cancer risk.
Tobacco carcinogens such as NNK can bind to DNA and form DNA adducts. Failure of the normal DNA repair mechanisms to remove DNA adducts can lead to permanent mutations. NNK can mediate signaling pathway activation that includes modulation of critical oncogenes and tumor-suppressor genes that promote uncontrolled cellular proliferation and tumorigenesis.36 NNK is associated with DNA mutations resulting in the activation of K-ras oncogenes.37,38 K-ras oncogene activation has been detected in 24% of human lung adenocarcinomas,39 and is present in adenocarcinoma of the lung in ex-smokers, suggesting that such mutations persist even after smoking cessation.40 This finding may explain the persistent elevation in lung cancer risk in ex-smokers even years after discontinuing cigarette use. Similarly, benzo(a)pyrene metabolite, a specific chemical constituent of tobacco smoke, can damage various p53 tumor-suppressor gene loci that are known to be abnormal in approximately 60% of primary lung cancer cases.41 Related PAHs in tobacco smoke can also target other lung cancer mutational hot spots.42
 OTHER TYPES OF SMOKING
OTHER TYPES OF SMOKING
Cigar smoking and pipe smoking have been associated with increased risk for lung cancer, although seemingly not as great a risk as with cigarette smoking. The tobacco content of cigars can vary from 1 g to 20 g. Smoking five cigars a day on average is equivalent to smoking one pack of cigarettes a day. Cigar smokers have a relative risk of lung cancer of 2.1 to 5.1 compared to nonsmokers; men who smoked five or more cigars a day have the greatest risk.43 The risk for lung cancer in pipe smokers is similar to that in cigar smokers.44,45 The effects of smoking marijuana and cocaine have not been extensively studied, and an association has not been fully established between such inhalant drug use and lung cancer. There have, however, been reports of increased risk for lung cancer in marijuana smokers; and metaplastic, histologic, and molecular changes similar to premalignant alterations have been detected in the bronchial epithelium in habitual smokers of marijuana or cocaine.46,47
 NEVER SMOKERS
NEVER SMOKERS
Never smokers are defined as persons who have smoked fewer than 100 cigarettes in their lifetime, including lifetime nonsmokers. An estimated 15% of lung cancers in men and up to 53% in women worldwide occur in never smokers, accounting for 25% of all lung cancer cases.48 Lung cancer in never smokers considered as a distinct group would rank as the seventh most common cause of cancer death worldwide, ahead of cervical, pancreatic, and prostate cancer.49 In countries in South Asia, up to 80% of women with lung cancer are never smokers.50 In the United States, one study estimated that 19% of lung cancers in women and 9% in men occur in never smokers.51 The age-adjusted rate for lung cancer in never smokers (aged 40–79 years) ranges from 11.2 to 13.7 per 100,000 person-years for men and from 15.2 to 20.8 per 100,000 person-years for women. The rates are 12 to 30 times higher in current smokers of the same age group.52
Adenocarcinoma of the lung is more common than squamous cell carcinoma in never smokers,50 and recent data suggests that adenocarcinoma is also becoming more common in smokers.53,54 Risk factors considered to be important for never smokers include environmental tobacco exposure (“secondhand smoke”); environmental exposures to carcinogens such as radon, outdoor and indoor air pollution, asbestos, and arsenic; a history of lung disease including interstitial lung disease; and genetic factors.55 A population-based case-control study in Canada found that occupational exposures, history of lung disease, and family history of early-onset cancer were important risk factors for lung cancer among never smokers.56 In this study, potential environmental sources of increased risk included exposure to solvents, paints or thinners, welding equipment, and smoke, soot, or exhaust. Other studies have implicated a genetic role in lung cancer in light of associations between lung cancer in never smokers and a family history of lung cancer.57–59 Genes implicated include the epidermal growth factor receptor (EGFR) gene, the human repair gene (hMSH2), and various cytochrome P450, and glutathione-S-transferase (GST) enzymes. A case-control study following 2400 relatives of 316 never smokers with lung cancer cases showed a 25% excess risk for cancer in first-degree relatives.58
The investigation threshold in symptomatic never smokers might be higher than in smokers, leading to diagnosis at later stages in never smokers.60 Despite this potential delayed diagnosis and later presentation of lung cancer in never smokers, the survival rate for never smokers is better than for smokers, independent of stage of disease, treatment received, and presence of comorbidities.61–63 A multivariate analysis of lung adenocarcinoma found that never smoking status was an independent predictor of improved survival (23% overall 5-year survival rate for never smokers; 16% for current smokers).61 Such findings have suggested that the cancer in never smokers may display a distinct biologic behavior and natural history. Microarray gene profiling studies have found that lung adenocarcinomas are heterogeneous, and the profiles of cancer in smokers and never smokers are quite different.64,65 In 2010, the first genome-wide association study (GWAS) reported genetic variations in never smoking females in chromosome 13q31.3 that altered the expression of glypican 5 (GPC5), a heparin sulfate proteoglycan with many known functions involving cell growth and differentiation and tissue responses.66 Another GWAS, focusing on lung adenocarcinomas in female Han Chinese never smokers in Taiwan, identified genetic variation in the CLPTM1L-TERT locus of chromosome 5p15.33 to be associated with risk for lung cancer in this population.67 This 5p15.33 chromosome contains two genes implicated in carcinogenesis, telomerase reverse transcriptase (TERT) and cleft lip and palate transmembrane 1-like (CLPTM1L).
 GENETIC FACTORS
GENETIC FACTORS
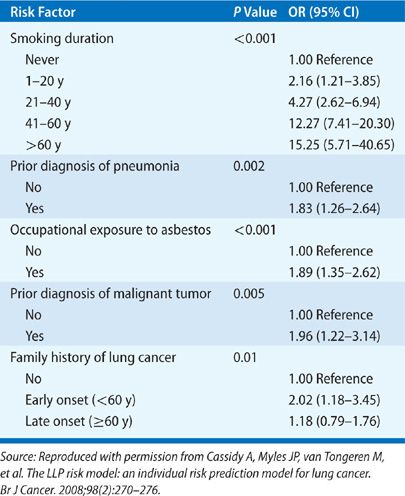
The genetic component of lung cancer relates to host susceptibility with or without exposure to cigarette smoke, the development of certain types of lung cancer, and the patient’s responsiveness to biologic therapies. A lung cancer risk prediction model developed by Spitz et al. incorporated such variables as smoking history, exposure to ETS, occupational exposures to dusts and to asbestos, and family history of cancer.68,69 Their analysis showed the influence of family history of cancer on the risk for lung cancer in never smokers, former smokers, and current smokers (Table 109-1). Cassidy et al.70 also highlighted a significantly increased risk for lung cancer specifically for persons with a family history of early-onset lung cancer (<60 years of age) (Table 109-2).
TABLE 109-1 Multivariable Logistic Model for Lung Cancer by Smoking Status
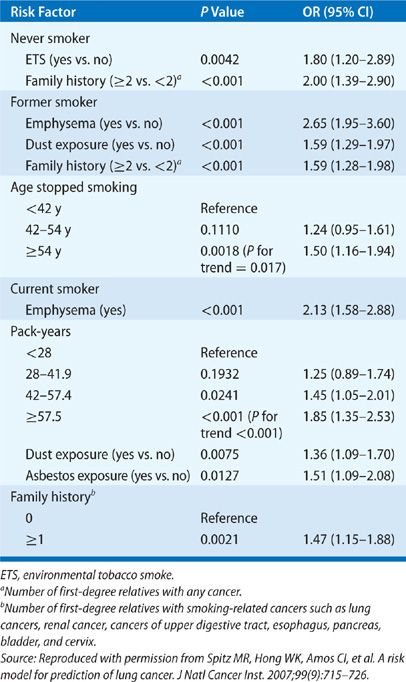
TABLE 109-2 Liverpool Lung Project – Multivariable Risk Model Lung Cancer
Recent reviews have examined the molecular epidemiology and biology of lung cancer, focusing on genetic markers of host susceptibility to lung carcinogens and their clinical implications.71,72 The susceptibility genetic factors include high-penetrance low-frequency genes, low-penetrance high-frequency genes, and acquired epigenetic polymorphisms. There are associations of lung cancer with rare Mendelian cancer syndromes such as Bloom and Werner syndromes. Familial aggregation studies have demonstrated a hereditary component to the risk for lung cancer and have been used to discover high-penetrance, low-frequency genes. There is a twofold increased risk for lung cancer in persons with a family history of lung cancer, with an increased risk also present in nonsmokers.73 There have also been numerous studies on candidate susceptibility genes that are of low penetrance and high frequency. The approach has been to target genes known to be involved in the absorption, metabolism, and accumulation of tobacco or other carcinogens in lung tissue. For example, genetic polymorphisms encoding enzymes involved in the activation and conjugation of tobacco smoke compounds, including PAHs, TSNAs, and aromatic amines have been widely studied. Some of the frequently studied enzymes in this system include CYP1A1, the GSTs, microsomal epoxide hydrolase 1 (mEH/EPHX1), myeloperoxidase (MPO), and NAD(P)H quinine oxidoreductase 1 (NQO1). A GWAS of tagged single nucleotide polymorphisms (SNPs) in histologically confirmed NSCLC was recently performed to identify common low-penetrance alleles that influence lung cancer risk. This study identified a susceptibility locus that contains the nicotinic acetylcholine receptor genes.74 Polymorphisms in genes involved in DNA repair enzymes active in base excision repair (XRCC1, OGG1), nucleotide excision repair (ERCC1, XPD, XPA), and double-strand break repair (XRCC3) and different mismatch repair pathways have been studied as they relate to lung cancer risks.75,76 Chronic inflammation in response to repetitive tobacco exposure has been theorized to be involved in lung tumorigenesis.77 Genes encoding for the interleukins (IL-1, IL-6, IL-8) or the cyclooxygenase enzymes (COX2) involved in inflammation, or the metalloproteases (MMP-1, -2, -3, -12) involved in repair during inflammation, and more recently NFKB1 have been associated with lung cancer risk.78 Various cell cycle–related genes have also been implicated in lung cancer susceptibility, including the tumor-suppressor genes p53 and p73, mouse double minute 2 (MDM2), and the apoptosis genes encoding FAS and FASL.79,80 Acquired or epigenetic changes to DNA chromosome can also lead to increased lung cancer susceptibility. These events include changes such as DNA methylation, histone deacetylation, and phosphorylation, all of which can affect gene expression.81 Despite numerous genetic association studies, the specific genes responsible for the enhanced risk for lung cancer have not been identified. Collaborative efforts such as the Genetic Susceptibility to Environmental Carcinogens and the International Lung Cancer Consortium are attempting to pool findings to achieve greater study sample sizes.82
 GENDER
GENDER
In the late 1980s, lung cancer surpassed breast cancer as the leading cause of cancer death in women in the United States. At present, nearly twice as many American women die annually of lung cancer than succumb to breast cancer.4 Since 1950 there has been a more than 600% increase in the lung cancer mortality rate in women. In the United States, the cigarette smoking rate for women increased during the period from 1930 to 1960; this increase was followed by an increase in lung cancer in women starting around 1960.83,84 Smoking prevalence is higher among men (23.1%) than women (18.3%), but the difference is narrowing.29 In 2009, lung cancer death rates for women declined for the first time in four decades, along with a decrease in the overall cancer death rate.5 This trend varies geographically; for example, the rate of lung cancer is increasing among women in southern and midwestern states.85
Whether women are more or less susceptible than men to the carcinogenic effects of cigarette smoke is controversial. While lung cancer in never smoking women is more common than in never smoking men, up to 80% of lung cancer cases in women are related to smoking.82 A recent study from the U.S. National Health Interview Survey showed a staggeringly high hazard ratio for lung cancer mortality of 17.8 for female smokers, compared to 14.6 for male smokers.14 Recent analysis of the SEER data from 1997 to 2006 showed that the lung cancer mortality rate is 74.08 per 100,000 man-years compared to 40.81 per 100,000 woman-years.86 However, other studies have suggested that women may be actually more vulnerable to carcinogens in tobacco smoke than men.87–90 A study using the American Health Foundation data found that the odds ratio for the major lung cancer types has been consistently higher for women than for men at every level of exposure to cigarette smoke.90 The dose-response odds ratios for lung cancer in women were 1.2- to 1.7-fold higher than in men. A Canadian case-control study of male–female differences in lung cancer covering the period 1981 to 1985 showed that with a history of 40 pack-years of cigarette smoking relative to lifelong nonsmoking, the odds ratio for women developing lung cancer was 27.9 versus 9.6 in men. The gender differences in susceptibility may be related to differences in nicotine metabolism and in metabolic activation or detoxification of lung carcinogens; women have higher levels of DNA adducts than men, which may result in greater susceptibility to carcinogens.91 Hormonal factors may also play a role in susceptibility. A case-control study showed that estrogen replacement therapy was significantly associated with an increased risk for adenocarcinoma (odds ratio 1.7), and the combination of cigarette smoking and estrogen replacement increased that risk substantially (odds ratio 32.4).92 Conversely, early menopause (age 40 years or younger) was associated with a decreased risk for adenocarcinoma (odds ratio 0.3). More recent large randomized studies suggest that the use of hormonal therapies such as estrogen and progestin may be associated with an increased risk of lung cancer in women.93
A separate but related issue is whether cigarette smoking may be associated with a higher risk of nonmalignant lung disease in women than in men. Neither the British Physicians Study in the United Kingdom94,95 nor the Lung Health Study in the United States96 found gender differences in mortality from smoking-related chronic obstructive pulmonary disease (COPD). However, other studies, including a report by Chen et al.,97 suggest that cigarette smoking may be more harmful to pulmonary function in women compared with men. In this study, changes in forced expiratory volume in 1 second (FEV1) and maximal midexpiratory flow rate increased with increasing pack-years more rapidly in women smokers than in their male counterparts. These changes were independent of age, height, and weight. Beck and colleagues in a study of 4690 Caucasians found that for a given level of smoking, women had greater decline in FEV1 and maximal expiratory flow at 25% and 50% of vital capacity at a younger age (15–24 years) than men (40–45 years).98 Because smokers with spirometric evidence of airway obstruction are at higher risk for lung cancer, the suggestion that women have increased susceptibility to smoking-induced airway disease may be important in the consideration of their risk for lung cancer.98
Finally, it also appears that lung cancer is more common in nonsmoking women than in nonsmoking men. For example, in never smokers, the age-adjusted incidence rate of lung cancer is much higher for women than men (14.4–20.8 per 100,000 person-years for women, compared with 4.8–13.7 per 100,000 person-years for men).51 The proportion of never smoking lung cancer patients was more than twice as high for women than for men in a case-control study.90
 RACE AND ETHNICITY
RACE AND ETHNICITY
Race is a complex variable that often has a strong socioeconomic association. However, notable racial differences in disease states can shed light on the specific issues of a particular subpopulation. In general, the incidence of lung cancer is substantially higher among blacks, Native Hawaiians, and other Polynesians, and lower among Japanese Americans and Hispanics than among whites in the United States.99 These differences initially have been attributed to the variations in cigarette smoking pattern among the different ethnic and racial groups. Recent smoking data show that among the different groups, Asians (9.9%) had the lowest smoking prevalence in the United States, whereas American Indians and Alaska Natives (32.4%) had significantly higher prevalence than the other groups.29 Smoking prevalence among whites (22%) and blacks (21.3%) was significantly higher than among Hispanics (15.8%). Black smokers have higher rates of lung cancer than white smokers, even though only 8% of black smokers smoked at least 25 cigarettes per day compared with 28% of white smokers.29 Native Hawaiians had higher rates of lung cancer than whites and Asians despite having similar smoking habits.29 The relative risk for lung cancer among subjects smoking less than 20 cigarettes per day were 0.21 to 0.39 for Japanese Americans and Latinos, and 0.45 to 0.57 for whites as compared with black Americans.29 However, the differences in lung cancer risks were not significant among all racial groups who exceeded 30 cigarettes per day of smoking. A recent SEER report specifically showed that black men, but not black women, in the United States had a higher age-adjusted incidence of lung cancer than their white counterparts at all age groups. Hispanics in the United States have lower incidence and death rates than non-Hispanic whites for lung cancer; however, they have higher rates for other organ cancers.100 Further, first-degree relatives of black persons with early-onset lung cancer have a much greater risk of lung cancer than their white counterparts (25.1% vs. 17.1%, respectively).101
The explanation for these racial or ethnic variations in risk for lung cancer is not known. Black Americans also have higher mortality rates from lung cancer than white Americans.29 This difference in mortality rates has been attributed not only to the higher incidence rates but also to the poorer survival of black patients with lung cancer than white patients. For example, the 5-year survival rate was 14.3% lower in black Americans compared to white Americans.29 The reasons for these racial differences are not known. Some have hypothesized a potential role for greater use of menthol cigarettes among black Americans than among white Americans (69% vs. 22%), or the deeper inhalation of menthol cigarettes compared to nonmenthol cigarettes.102
 AGE
AGE
Although smoking prevalence is lowest among persons aged 65 years and older (9.3%) compared to persons aged 18 to 24 years (21.4%), 25 to 44 years (23.7%), and 45 to 64 years (22.6%),29 more than 65% of patients with lung cancer are older than 65 years.3 Specifically, 31.1% of patients with lung cancer are between 65 and 74 years; 29% between 75 and 84 years; and 8.3% are 85 years old and older.3 The mean age at the time of diagnosis is 71 years old. This difference between relatively lower current smoking prevalence and the higher cancer rate in the elderly population likely reflects the effects of prior smoking. In the past decade, the incidence and mortality from lung cancer have decreased among persons aged 50 years and younger, but have increased among persons aged 70 years and older.103 The 5-year survival rate for lung cancer decreases incrementally with age for both sexes. Patients older than 80 years constitute 14% of all patients with lung cancer in the United States but account for almost a quarter of all lung cancer deaths.103 It has been estimated that the number of lung cancer patients aged 85 years and older will quadruple by 2050.104
 DIET AND OBESITY
DIET AND OBESITY
It has been suggested that diet is responsible for approximately 30% of all cancers.105 For example, low serum concentrations of antioxidants, such as vitamins A, C, and E, have been associated with the development of lung cancer.106,107 The carotenoid component of vitamin A, in particular β-carotene, has been shown to have protective effects against lung cancer in dietary studies. Vitamins C and E (α-tocopherol) have also been shown to have some protective effect.108,109 Based on those observations, several large intervention trials have been conducted to examine the relationship between vitamin supplementation and lung cancer. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study (daily supplementation of a-tocopherol, β-carotene, or both) and the Beta-Carotene and Retinol Efficacy Trial (CARET) (β-carotene, vitamin A, or both) found that vitamin supplementation did not reduce lung cancer risk, and in some circumstances, actually increased the incidence of lung cancer.110,111 Therefore, the use of supplemental β-carotene and vitamin A is discouraged. There have also been suggestions that low dietary intake of certain minerals, including magnesium, zinc, copper, and iron, is associated with increased lung cancer risk; however, a prospective cohort study observed no significant associations between total mineral intake and lung cancer risk.112,113
A diet rich in fruits and vegetables has been linked to decreased cancer incidence, and the protective effects are stronger in current than in former smokers.114 Although no specific type of vegetable or fruit has been shown to be particularly responsible for the effect, consumption of cruciferous vegetables, such as broccoli and cabbage that are rich in isothiocyanates, has some protective effect against lung cancer.115 Low or no intake of fruits or vegetables has been associated with up to threefold risk for lung cancer.116 It has been also further suggested that consuming fruits or vegetables raw rather than cooked is associated with a further reduction in risk for lung cancer because important carotenoids can be destroyed with cooking.117 A large prospective NIH-AARP Diet and Health Study showed no relation between total intake of fruit and vegetables with lung cancer risk.118 However, the study showed that higher consumption of several botanical groups such as rosaceae (apples, peaches, strawberries), convolvulaceas (sweet potatoes and yams), and umbelliferae (carrots), was significantly and inversely associated with lung cancer risk in men and in former smokers.118 Flavonoid plant metabolites have antioxidant and antiproliferative properties and can be found in food such as berries, citrus fruits, tea, dark chocolate, and red wine. A prospective study showed that the risk for lung cancer was lower in men with the highest total flavonoid intake compared to those with the lowest intake.119 Certain dietary items including red meat, dairy products, saturated fats, and lipids, have been reported to be associated with an increased risk of lung cancer.120–123 Other foods found to have an adverse effect on lung cancer risk include items that contain nitrosodimethylamines and nitrites, such as those found in salami and salted and smoked meat products.124,125
In the United States, 35.1% of adults are classified as obese.126 Excessive body weight has been associated with increased risk for endometrial, breast, and colorectal cancers but not for lung cancer. A meta-analysis reported that an inverse association between body mass index (BMI) and lung cancer risk, and obesity may even have a protective role.127 However, in the absence of cigarette smoking, the association between BMI and lung cancer was not significant. It has been proposed that the observed BMI and cancer association may be related to residual strong confounding effects of smoking itself.128 For example, smokers tend to have lower mean BMI than age- and sex-matched nonsmokers.129 Smokers have a notably lower BMI than nonsmokers, and they gain weight when they quit smoking. More recent studies that adjusted for pack-years of smoking and other relevant covariates in a female cohort showed an inverse association of BMI and lung cancer risk in current and former smokers, whereas BMI was positively associated with lung cancer in never smokers.130 Other studies have shown that waist circumference was positively associated with lung cancer risk in smokers.131
OTHER LUNG DISEASES AND AIRWAYS OBSTRUCTION
Some nonmalignant lung diseases have been associated with an increased risk for lung cancer, the strongest association being with COPD. Cigarette smoking is the primary cause of both lung cancer and COPD. COPD affects an estimated 40% to 70% of patients with lung cancer, a finding that reflects a common smoking history. Some evidence suggests an association between the presence of airflow obstruction and the development of lung cancer.132 A recent study evaluated 602 patients with lung cancer and found that 50% of them had prebronchodilator pulmonary function test results consistent with a diagnosis of COPD GOLD stage 2 and higher, independent of age, sex, and smoking history, with an odds ratio of 11.6.133 The prevalence of COPD in patients with newly diagnosed lung cancer was sixfold greater than matched smokers, suggesting that COPD itself is an important independent risk factor with potential relationship to the pathogenesis of lung cancer.
COPD is characterized by chronic inflammation, which, in turn, has been suggested as a risk factor for lung cancer. A Dutch study found that the likelihood of developing lung cancer was increased in patients with an elevated serum C-reactive protein, a marker of generalized inflammation. A large retrospective study of patients with COPD found that the risk for lung cancer was lower in patients who took high-dose inhaled corticosteroids than in patients taking lower doses or none at all.134 These results suggest that inhaled corticosteroids may have a chemoprotective role in lung cancer in patients with COPD. A study of α1-antitrypsin deficiency carriers found that they have an approximately twofold higher risk for lung cancer, after adjusting for the effects of tobacco smoke exposure and COPD.135
The incidence of lung cancer has been demonstrated to be increased in patients with pulmonary fibrosis, even after adjustment for smoking.136 In a population-based cohort study, patients with pulmonary fibrosis had an odds ratio for lung cancer of 8.25 compared with control subjects. Other fibrosing diseases, including asbestosis and scleroderma-related lung disease, also appear to be associated with an increased risk of lung cancer.136,137 The mechanism by which pulmonary fibrosis may predispose to pulmonary malignancy is not clear.
INFECTIONS
Oncogenic viruses have been proposed as a cause of lung cancer. The possible involvement of human papillomavirus (HPV), which is known to cause carcinoma in other tissues, in bronchial squamous cell lesions was first suggested because the epithelial changes in bronchial carcinomas resemble those of established HPV condylomatous lesions in the female genital tract.138 HPV DNA has been detected in lung squamous cell carcinoma tissues.139 However, there is inconsistency in the reported prevalence of HPV infection in patients with lung cancer in different countries, with racial and geographic variations. Studies to date testing lung cancer specimens for HPV have yielded mixed results because of variability in genetic susceptibility, method of HPV detection, and environmental and high-risk behavior variables. Epstein–Barr virus (EBV), which is associated with Burkitt lymphoma and nasopharyngeal carcinoma, has been strongly associated with lymphoepithelioma-like carcinoma (LELC), a rare form of lung cancer in Asian patients, but this association has not been observed in the Western population.140 Other viruses suggested as etiologic for lung cancer include BK virus, JC virus, the human cytomegalovirus, simian virus 40 (SV40), and measles virus; however, the evidence of causality is inconclusive.141–144 More recently, DNA from Torque teno virus (TTV), a relatively new virus, has been detected at high levels in idiopathic pulmonary fibrosis patients with lung cancer, but more studies are needed to confirm these findings and determine their clinical significance.145 It has also been suggested that Chlamydia pneumoniae, a common cause of acute respiratory infection, especially in patients who smoke cigarettes, might be involved in lung carcinogenesis.146 If such an association were established, it could have profound implications, particularly for lung cancer prevention. Chlamydia is not a known oncogenic pathogen, but some have hypothesized that the inflammation resulting from the infection can lead to reactive oxygen species that damage DNA and cause cell injury, resulting in mutations that may lead to an increased risk of tumorigenesis.
Some studies have also reported an association of pulmonary tuberculosis with lung cancer.147,148 A cohort study showed an increased risk for lung cancer in tuberculosis patients with hazard ratio of 3.3 after adjusting for confounding factors such as COPD and smoking-related cancers other than lung cancer. The effect of tuberculosis was even greater when combined with COPD or with other smoking-related cancers.148 Some have speculated that tuberculosis-related inflammation and scarring contribute to lung cancer pathogenesis.147
AIDS-related mortality has dramatically decreased since the advent of highly active antiretroviral therapy; however, this decrease has been accompanied by an increase in the proportion of deaths attributable to non-AIDS defining tumors, especially lung cancer.149,150 The increased risk of lung cancer relative to the general population of the same age seems to be due in part to the higher prevalence of smoking among HIV-infected patients. HIV was associated with a hazard ratio of 3.6 for lung cancer after controlling for smoking status.151 Although smoking is a key risk factor for lung cancer in HIV-infected patients, several other factors may contribute to the higher incidence of lung cancer. These include greater prevalence of coinfection with oncogenic viruses such as human herpesvirus 8, HPV, and EBV and the potential direct effects of the HIV virus and the consequences of long-term immunosuppression.152 For example, the HIV tat protein can transactivate cellular genes or proto-oncogenes, while other HIV genes inhibit tumor-suppressor genes.153 HIV-infected patients with lung cancer have a worse prognosis than similarly staged non–HIV-infected patients.154 They are also more likely to have more advanced stage lung cancer at diagnosis.155 Studies have reported that HIV-infected patients were much younger, were more likely to be smokers, and had significantly reduced median survival.155,156
ENVIRONMENTAL TOBACCO SMOKE
ETS, also referred to as “secondhand smoke,” contributes to an increased risk for lung cancer with a notable dose-dependent relationship.157 ETS consists of both mainstream (exhaled) smoke and sidestream smoke. In one study, household exposure of 25 or more smoker-years before adulthood doubled the risk for lung cancer; exposure of less than 25 smoker-years did not increase risk.158 At least 17% of lung cancers in nonsmokers are thought to be attributable to exposure to high levels of ETS during childhood and adolescence.158 The Surgeon General’s 1976 report raised concerns about hazards relating to such environmental smoke exposure.159 Nonsmokers exposed to ETS have an increased rate of smoke-related problems, including upper respiratory symptoms and eye irritation, and exposed children have an increased frequency of respiratory illnesses. Therefore, the report suggested that the acknowledged carcinogenic effect of active tobacco smoking might also be present in those involuntarily exposed. The risk for lung cancer is increased in nonsmoking women married to men who smoke.160,161
The risk of lung cancer related to ETS has been studied, though quantification of the magnitude of exposure is problematic. In an analysis of 37 epidemiologic studies of the risk of lung cancer in nonsmokers who did or did not live with a smoker, encompassing 4626 cases, a lifetime nonsmoker had an estimated 24% greater risk of lung cancer if he/she lived with a smoker.157 It is important to note that this should be interpreted from the perspective that the background risk for lung cancer in a nonsmoker is very low, and so a 24% increase will not substantively change that risk.157 Similarly, in 1986, the National Research Council commissioned a review of the effects of ETS as a potential causal agent of lung cancer in nonsmokers exposed to household cigarette smoke.162 Review of all the available evidence yielded an overall odds ratio of 1.34 in lung cancer risk associated with ETS. In nonsmokers, this translates into an approximately 30% increase of risk for lung cancer, which, as already noted, should be interpreted in the context of a low background risk.162
Many government agencies, including the U.S. Department of Health and Human Services, Environmental Protection Agency, and the IARC, classify ETS as containing lung carcinogens. The presence of ETS is pervasive and harmful. There are reports that suggest as many as 88% of nontobacco users have detectable levels of serum cotinine, a metabolite of nicotine, presumably from exposure to ETS.163 Therefore, efforts to limit public smoking will be of great benefit in this regard. Given that 20.6% of the American adult population still smoke, ETS will continue to be a major public health issue until cigarette smoking altogether is eliminated.164
ENVIRONMENTAL POLLUTION
Outdoor air pollution has long been thought to increase the risk for lung cancer. Advances in analytical methods used to detect specific pollutants have helped investigators study the effects of airborne particulates. Early studies involving urban–rural comparisons have shown that an “urban factor” is associated with a 10% to 40% increase in lung cancer deaths.165 Two large US cohort studies suggest that there is an excess risk for lung cancer of about 19% per 10 μg/m3 increment in the long-term average exposure to fine particulates, after adjustments for multiple confounding factors. The Cancer Prevention II study found that fine particulate and sulfur oxide–related pollution were associated with 8% increased risk for lung cancer mortality for each 10 μg/m3 elevation in long-term average ambient concentration of fine particles less than 2.5 μm in diameter.166
However, it is difficult to determine the carcinogenicity of single constituents of air pollution. Other sources of fossil fuel combustion products can also have potential carcinogenic components. Estimates of relative risks for lung cancer associated with exposure to combustion products range from 7.0 to 22.0 in cigarette smokers, 2.5 to 10.0 in coke oven workers, and 1.0 to 1.6 in residents of areas with high levels of air pollution.167 Diesel exhaust, which is composed of a complex mixture of gases and fine particles, is also an important component of air pollution. Some of the gaseous components of diesel exhaust, including benzene, formaldehyde, and 1,3-butadiene, are suspected of causing or known to cause cancer in humans. There is strong support that occupational exposure to diesel exhaust, particularly in persons in the trucking industry, is associated with an approximately 30% to 50% increase in the relative risk for lung cancer.168 Data linking gasoline engine exhaust and lung cancer are less compelling. In many parts of the world, solid fuels such as wood are burned as primary sources of domestic energy for cooking and heating. Incomplete combustion of coal in homes in China has been linked with lung cancer.169 The IARC has classified indoor emission from household coal combustion as a human carcinogen and emissions from biomass fuel, primarily from wood, as a probable human carcinogen.
OCCUPATIONAL CARCINOGENS
The IARC has identified arsenic, asbestos, beryllium, cadmium, chloromethyl ethers, chromium, nickel, radon, silica, and vinyl chloride as carcinogens. The occupations associated with exposure to these agents are shown in Table 109-3. It has been estimated that 10% of lung cancer deaths in men and 5% in women worldwide could be attributable to exposure to eight occupational lung carcinogens, namely asbestos, arsenic, beryllium, cadmium, chromium, nickel, silica, and diesel fumes.170,171 Worldwide, lung cancer related to occupational carcinogen exposures is estimated to be responsible for 152,000 deaths, and a loss of nearly 1.6 million disability-adjusted life years (DALYS).170 The National Institute for Occupational Safety and Health (NIOSH) estimated that approximately 9000 to 10,000 men and 900 to 1900 women per year in the United States develop lung cancer from exposure to occupational carcinogens.172
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree