Epidemiology of heart failure
Despite the considerable burden of heart failure (HF), its epidemiology is still poorly defined, especially in primary care.1 A precise analysis of the epidemiology must take into account the difficulty in defining the syndrome itself, particularly in a predominantly elderly population in whom symptoms and signs are less specific. An ideal estimate of the true epidemiology would be based on surveys in random samples of the general population, using validated questionnaires and targeted physical examinations in conjunction with objective measures of left ventricular systolic dysfunction (LVSD) such as imaging and possibly supported by validated biomarkers. Broadly speaking, contemporary studies can be divided into those analyzing the prevalence and incidence of “symptomatic” HF (further divided into those with preserved and reduced LV ejection fraction [LVEF]) and those investigating the prevalence of LVSD (of which a significant proportion of patients will be asymptomatic).
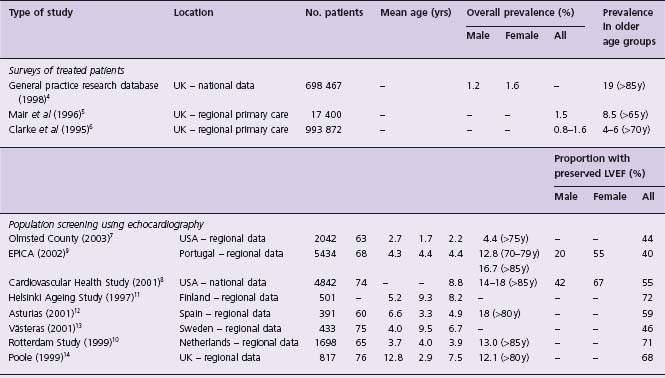
Prevalence of symptomatic HF (Table 46.1)
Studies utilizing a range of designs suggest that the prevalence of HF is around 2–5% of the population of the developed world, increasing considerably with age. Whilst age-adjusted incidence has remained stable in the last two decades, prevalence may be increasing.2 Furthermore, approximately half of all patients with symptomatic HF have preserved LVEF.3 Prevalence varies widely from 0.4% to 19% in older age groups based on general practice (GP) studies in the UK. The GP Research Database, involving 211 general practices in England and Wales (representing 2.6% of the national population), reported an overall prevalence (physician diagnosis) of 12.2/1000 in men and 15.8/1000 in women, increasing to 190/1000 in those over 85 years.4 The substantial increase in prevalence with aging has been confirmed in additional UK primary care studies.5,6
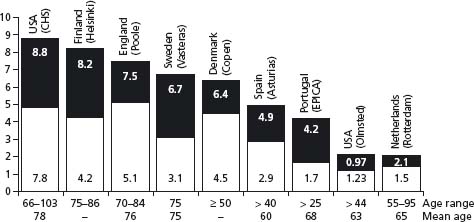
Several population-based observational studies have been undertaken in North America and continental Europe (see Table 46.1). The prevalence of symptomatic HF in the USA is reported to be of the order of 2.2% and similarly increases considerably with age.7,8 The European EPICA study of 5434 individuals attending general practices in Portugal reported that prevalence was 44.6/1000 in those >25 years and increased noticeably in the elderly.9 These data are supported by contemporary data from across Northern and Southern Europe.10–14 A significant proportion of patients with symptomatic HF have preserved LVEF. In the Olmsted County Study, 55% of symptomatic patients had preserved LVEF, an observation which is consistent with several other epidemiologic studies (Fig. 46.1).3
Figure 46.1 Prevalence of heart failure in cross-sectional, population-based and echocardiographic studies. Black bars show percentage prevalence; lower portion of bars shows the proportion of cases associated with preserved systolic function. LV, left ventricular. (Reproduced with permission from Hogg et al. 3)
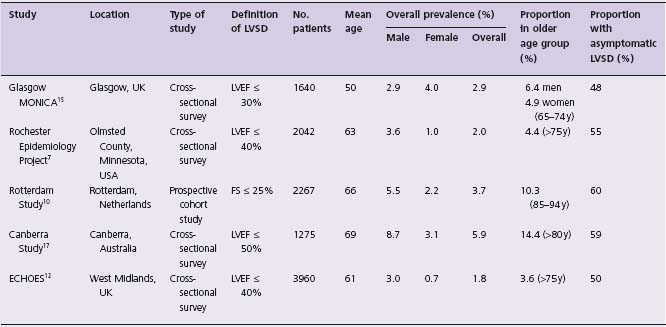
Prevalence of LVSD (Table 46.2)
Table 46.2 Reported population prevalence of LVSD
LVEF, left ventricular ejection fraction; FS, fractional shortening.
A number of studies have used an objective measure of LVEF to estimate the population prevalence of LVSD. The Glasgow MONICA Study included 1640 subjects aged 25–74 who were assessed by questionnaire, electrocardiography and transthoracic echocardiography (TTE).15 Prevalence of LVSD (LVEF < 0.30) was 2.9% and increased with age. A cohort of 2267 subjects from the Rotterdam Study (which used a combination of clinical criteria and prescribing patterns to define HF) underwent echocardiography.10 Prevalence of LVSD (defined by fractional shortening <25%) was 5.5% in men and 2.2% in women. Similar data come from the UK, USA and Australia.7,16,17
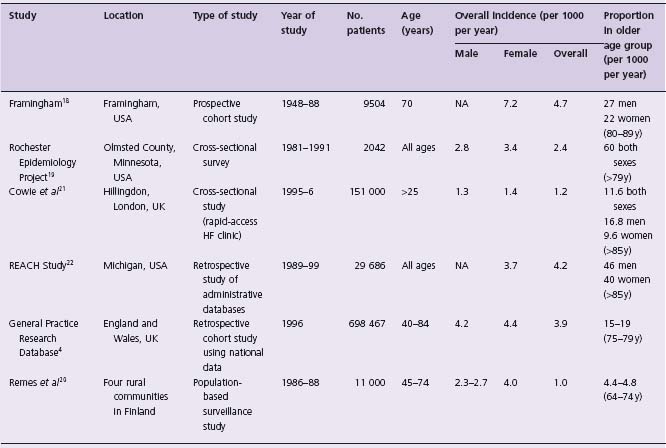
Incidence of symptomatic HF (Table 46.3)
An estimate of the incidence of HF is more difficult to define, but there are considerable data, particularly from large population-based studies. In the Framingham Heart Study, at 34 years follow-up, incidence was approximately 2/1000 person-years in subjects aged 45–54, increasing to 40/1000 in men aged 85–94.18 Similar rates are reported from the Olmsted County Study and UK and Finnish population studies.4,19,20 In the UK, Cowie and colleagues reported incidence in referrals from primary care. They identified new cases from a population of 151 000 in London, through surveillance of hospital admissions and through referrals to a rapid-access HF clinic.21 Diagnosis of HF was determined by a panel of cardiologists and supported by echocardiography. Incidence was 1.3/1000 overall for those ≥ 25 years. Incidence increased with age and was higher in men than women. Overall, only 29% of patients referred had an adjudicated final diagnosis of HF, illustrating the difficulty of classification on the basis of clinical assessment alone.
The age-adjusted incidence of HF appears to be stable. In the Resource Utilization Among Congestive Heart Failure (REACH) Study, which was a retrospective study using administrative databases of outpatient visits and hospitalizations in Michigan, USA, the incidence of HF in 1999 was 3.7/1000 person-years in men and 4.2/1000 person-years in women of all ages with no changes between 1989 and 1999.22 In a similar analysis of the Fram-ingham Heart Study, incidence was examined in four periods: 1950–69, 1970–79, 1980–89 and 1990–99.18 When compared with the rate for the period from 1950 to 1969, incidence remained virtually unchanged among men in the three subsequent periods but declined by 31–40% among women.
Incidence of HF with preserved ejection fraction
The body of evidence suggests that approximately 40–50% of patients have preserved EF (HF-PEF). An analysis of the Olmsted County Study identified 6076 patients with incident HF identified from the general population between 1987 and 2001 and suggested that about half had preserved EF (47%) whereas studies in patients hospitalized with HF suggest lower proportions (e.g. 31% in a study undertaken in Ontario, Canada).23,24 The EuroHeart Failure Survey reported on the characteristics and outcome of 6806 patients admitted to 115 European hospitals with HF between March 2000 and May 2001.25 Forty-six percent of patients had HF-PEF (LVEF ≥ 40%); these patients were more likely to be older, hypertensive and have a history of chronic atrial fibrillation, whereas coronary artery disease (CAD) was more prevalent in the systolic HF group (P < 0.001). The observed variations in the reported proportion of HF-PEF in these studies are partly explained by the differing thresholds for differentiating HF-PEF from systolic HF and the proportion of patients undergoing quantitative assessment of LVEF. Nevertheless, these data indicate that a significant proportion of patients have HF-PEF.
HF morbidity: hospitalizations
Despite the limitations of administrative databases, there is evidence that age-adjusted hospitalization rates have significantly increased in the developed countries over the last two decades (Fig. 46.2), emphasizing the growing global nature of this problem.1 In the USA and UK, HF is the most common cause of hospitalization in people aged ≥65 years.26,27 In Scotland, the number of hospital admissions for HF increased by 16% in men and 12% in women between 1990 and 1996, with some apparent plateauing by 1993–94.28 Similar data have been reported in The Netherlands.29
Figure 46.2 Comparison of heart failure admissions rates per annum (recorded hospital admission/10 000 population at risk) in Western developed countries, 1978–1993. (Adapted with permission from data in McMurray et al, Heart 2000;83:596–602.)
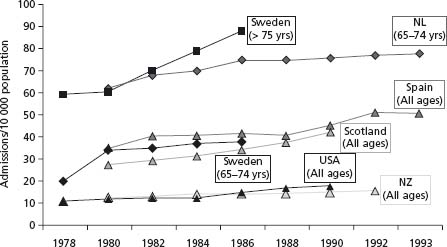
Men tend to be younger than women at index admission, but because of greater female longevity, overall admission numbers are roughly equal. The NHS Heart Failure Survey determined that 50% of patients with admissions for HF were male but that female patients were older (mean 80 v 75 years, P < 0.001).30 Overall, the typical HF patient is getting older. In The Netherlands between 1985 and 1995, mean age rose from 71.2 to 72.9 years in men and from 75.0 to 77.7 years in women.30 Elderly patients are more likely to have prolonged admissions, be managed by non-specialists, and be burdened by co-morbid conditions prolonging their admission.31 An admission to hospital with HF is frequently prolonged and in many cases followed by readmission within a relatively short time, particularly amongst the very elderly. In England and Wales, mean length of stay is 7 (IQR [interquartile range] 4–14) and 8 (IQR 4–14) days for men and women respectively.31 Transfer to a non-specialist unit adds another 2 days. Readmission to hospital is both common and costly. In Scottish hospitals in 2002, the average 30-day readmission rate was 5.3% (lowest 3.3%, highest 7.3%) and may be even higher in the USA.26,32
HF mortality
HF prognosis remains poor despite considerable therapeutic advances. However, official statistics continue to specifically attribute only a small proportion of deaths to HF. This reflects a common policy of coding the cause of death as the underlying etiology (e.g. CAD) rather than as HF itself. Population level data suggest that HF-related mortality is comparable to that of cancer; in the Framingham Heart Study the 5-year mortality was as high as 75% in men.33 Similarly, in a UK study, 1-and 5-year mortality following a first hospitalization was 43% and 73% respectively, with increasing relative risks with increasing age.34 In Scotland between 1986 and 1995, crude mortality rates in incident HF were 19.9% at 30 days, 44.5% at 1 year and 76.5% at 5 years.35 Median survival was 1.47 years in men and 1.39 years in women.
In the Rotterdam Study, survival rates from prevalent cases of HF were more favorable, with 1-and 5-year survival rates of 89% and 59% respectively.36 This still, however, represents a threefold increase in the age-and gender-matched risk of death compared to the general population. However, prognosis appears to be improving. In 2000, MacIntyre et al demonstrated a fall in 30-day case fatality rates by 26% in men and 17% in women between 1986 and 1995.35 Similar reductions were seen with longer term mortality. In the Framingham Study, 1-and 5-year mortality rates in men declined from 30% to 70% in 1950–69 and to 28% and 59% in 1990–99, with similar decreases in women.33 In the Olmsted County Study, age-adjusted 5-year survival improved from 43% in 1979–84 to 52% in 1996–2000. Similar compeling reductions in mortality were identified in data from nearly 300 000 hospitalizations from the Swedish Hospital Registry between 1988 and 2000.37 These improvements in outcome temporally correlate with the emergence of evidence-based therapies offering potential incremental benefits.
Prognosis of HF-PEF
With respect to hospitalization rates, in the Olmsted County Study, 24% of patients with HF-PEF were never hospitalized, 51% hospitalized once, and 25% hospitalized more than twice over a 5-year period.38 Patients with systolic HF had significantly more hospitalizations (41% once and 49% more than twice over the same 5-year period). In hospital cohort studies, the reported rates of rehospitalization are similar between those with low and preserved EF. In the aforementioned Canadian EFFECT Study, there was no significant difference in either 30-day (4.5% v 4.9% for HF-PEF and systolic HF respectively, P = 0.66) or 1-year readmission rates (13.5% v 16.1% for HF-PEF and systolic HF respectively, P = 0.09) in patients who survived the index HF admission.39
Similarly, in the EuroHeart Failure Survey, rates of early (within 12 weeks) readmission were almost identical (and impressively high) between both groups (22% and 21% for HF-PEF and systolic HF respectively, P = 0.47), indicating that, regardless of LV EF, approximately one in five patients may experience an early (and costly) readmission to hospital.25
In terms of mortality, in the Framingham Study, patients with systolic HF had an annual mortality rate of 18.9% compared to 4.1% in age-and sex-matched controls with HF-PEF (over 6.2 years follow-up).40 Median survival was 4.3 years in those with systolic HF and 7.1 years in those with HF-PEF. A contemporary insight into comparative prognosis comes from the Candesartan in Heart Failure Reduction in Mortality (CHARM) Study which enrolled 7601 patients with symptomatic HF (over 25% of whom had preserved LVEF). The CHARM investigators related baseline LVEF to outcome in 7599 patients with symptomatic HF (of whom 55% were receiving a beta-blocker) randomized to receive candesartan or placebo.41 Over a median follow-up of 38 months, the hazard ratio (HR) for all-cause mortality increased by 39% for every 10% reduction in LVEF below 45% (HR 1.39; 95% confidence interval (CI) 1.32–1.46).
In contrast to the aforementioned population-based and clinical trial data, two contemporary hospital-based cohort studies suggest that there is little difference in outcome between these patient groups. In the Canadian EFFECT Study, both 30-day (7.1% v 5.3%, P = 0.08) and 1-year mortality (25.5% v 22.2%, P = 0.07) rates were broadly similar between systolic HF and HF-PEF respectively.39 Similarly, 5-year survival rates were only marginally better in those with HF-PEF in the Olmsted County Study (HR 0.96, 95% CI 0.93 –1.00, P = 0.03).23 It is difficult to accurately explain the differences between these data and those observed in the CHARM population which may suggest the need for care in extrapolating clinical course from trials to the general population.
Epidemiology of HF in the developing world
Data describing the epidemiology of HF in the developing world are scarce and are somewhat limited to single-center hospital-based studies. This reflects the relative difficulty of conducting large-scale studies in communities with finite resources. Furthermore, studies often lack objective measures of LV function and accurate determinants of etiology principally because of inaccessibility to specialist tests. Whereas cardiovascular disease is the principal cause of death in the developed world, it represents only around a quarter of deaths in the developing nations. In contrast, whereas mortality rates have generally declined in the developed world, cardiovascular events are accelerating elsewhere (particularly Asia), in parallel with the emergence of industrialization, “Western” lifestyle and greater longevity.42 This is often referred to as “epidemiologic transition”. Current projections predict that in the next two decades, cardiovascular disease (and undoubtedly associated HF) will emerge as the dominant cause of death in these regions.
In the early 1980s a case series of 315 HF patients admitted to a regional hospital in Nigeria suggested that HF accounted for 7% of all admissions.43 Almost half were deemed attributable to “cardiomyopathy” (in many cases undefined) and 10% due to rheumatic heart disease. There was also regional variation between Northern and Southern Nigeria, where hypertension accounted for 13% and 35% of cases respectively. Accurate analyses of the contribution of coronary disease were lacking. In contrast, in a small single-center case series undertaken in Kenya in 1999, HF accounted for only 3.3% of hospital admissions.44 Rheumatic heart disease was attributed in 32% of cases followed by undefined cardiomyopathy in 25%. Only 2.2% of cases were attributed to coronary disease (the assumption made in the presence of ECG features of previous myocardial infarction).
The Heart of Soweto Study mapped the emergence of cardiovascular disease in a geographically stable impoverished population in South Africa.45 A total of 1593 cases of newly diagnosed cardiovascular disease were identified during 2006. HF was the most common primary diagnosis (44%), of whom 53% had moderate-to-severe LVSD. Approximately one-third (35%) were attributed to cardiomyopathy, a further third (33%) to hypertension and only 9% to ischemic etiology and 8% to primary valvular disease (predominantly rheumatic heart disease). Nevertheless, 59% of all patients had modifiable cardiovascular risk factors, which could influence future risk and illustrates the changing epidemiology in this region.
Profound epidemiologic transitions have taken place in many parts of the Middle East, Central and Southern America and Asia with a resultant increase in the burden of cardiovascular disease. In Oman between 1992 and 1994, the prevalence of HF was 5.17/1000.46 Ischemic heart disease was the most commonly attributed cause (51.7%) followed by hypertension (24.9%) and idiopathic cardiomyopathy (8.3%). Overall, only 15.7% of patients had HF-PEF (defined as LVEF ≥50%).
In Central and Southern America several case series indicate that the incidence of HF is higher (up to 9.4% of medical admissions in one Brazilian study) and often attributed to modifiable causes, particularly CAD (around a third) and hypertension (around 20%).47,48 The relative contribution of Chagas’ disease varied between 4% and 20%. In Asian countries such as China, India and Malaysia, cardiovascular disease is now the leading cause of death and in a similar fashion to the West, CAD and hypertension have now replaced rheumatic valve disease as the dominant cause of HF which accounts for up to 6% of medical admissions.49–51
Epidemiology of asymptomatic left ventricular systolic dysfunction
Prevalence of asymptomatic left ventricular systolic dysfunction (ALVSD)
In a systematic review, Wang and colleagues identified 11 studies estimating the prevalence of ALVSD.52 After adjustment for factors including the definition of LVSD, they suggested 3–6% as a reasonable estimate. Prevalence appears to be 2–8-fold greater in men than in women, and increases significantly with age. Therefore, the prevalence of ALVSD is at least twice that of symptomatic HF.
Prognosis of ALVSD
Randomized controlled trials provide the largest source of data on prognosis in ALVSD but are limited by their under-representation of the “average” patient. In the Survival and Ventricular Enlargement (SAVE) trial of post-myocardial infarction (MI) LVSD, 13% of patients developed overt HF during median follow-up of 37 months.53 Similarly, during a median 3-year follow-up, 16% of the placebo-treated patients in the Study of Left Ventricular Dysfunction-Prevention (SOLVD-P) died, with 5% dying within the first year following randomization.54 Nearly one-third progressed to overt HF and 5% died suddenly. Mortality and HF risk were associated with the degree of baseline systolic dysfunction (mean LVEF was 28%). Several population-based observational studies have confirmed that ALVSD is associated with an increased risk of cardiovascular mortality, all-cause mortality and non-fatal cardiovascular events, including the development of symptomatic HF.55
Heart failure is a classic illustration of the cardiovascular disease continuum, where multiple overlapping mechanisms are involved in disease progression and the disease progressively worsens, providing the opportunity to influence the process. The greatest opportunity for reducing the incidence and excessive mortality and morbidity of HF is through preventive strategies. The ACC/AHA staged approach recognizes that there are established risk factors and behaviors known to be associated with increased risk (Fig. 46.3). Interventions designed to interrupt these processes could slow or halt disease progression and are therefore potential preventive targets. Table 46.4 illustrates the common risk factors identified as preventive targets in the ACC/AHA and CCS guidelines.
Figure 46.3 ACC/AHA guidelines for the evaluation of chronic HF: evolution of HF by stage. EF, ejection fraction; FHx CM, family history of cardiomyopathy; LVH, left ventricular hypertrophy. (Reproduced with permission from the American College of Cardiology Foundation.)
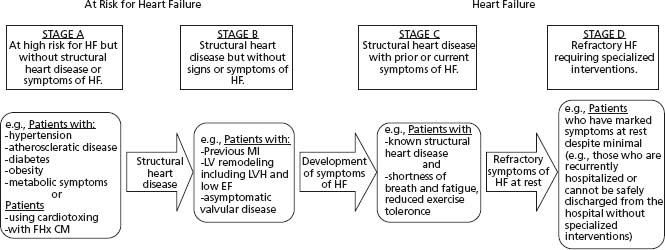
Table 46.4 Risk factors for the development of heart failure
| ACC/AHA Stage A (“at risk”) | ACC/AHA Stage B (“structural heart disease”) |
Hypertension * | Previous myocardial infarction |
Atherosclerotic disease* (especially coronary artery disease) | LV remodeling |
Diabetes mellitus * | Left ventricular hypertrophy |
Hyperlipidemia* | Left ventricular systolic dysfunction (asymptomatic) |
Smoking* | Asymptomatic valvular heart disease (especially mitral and aortic regurgitation) |
Obesity Older age Male sex Physical inactivity Heavy alcohol use Valvular heart disease (especially rheumatic valve disease) Chronic arrhythmia Infection Chagas’ disease HIV Enteroviruses (especially Coxsackie B) Thyroid dysfunction Drug induced Anthracyclines Cyclophsophamide Mitoxantrone Paclitaxel Trastuzumab Amphetamine abuse Cocaine abuse |
* Most important targets for prevention.
LVSD progresses gradually, often beginning with an index myocardial injury (such as MI) which leads to a progressive loss of functioning myocytes. The loss of cardiac function occurs as a consequence of ventricular remodeling, a process by which ventricular shape, dimension and function are altered. Remodeling consists of a multitude of maladaptive pathophysiologic processes including myocyte hypertrophy, necrosis and apoptosis and myocardial interstitial fibrosis and is exacerbated by activation of neurohormonal and inflammatory pathways.56 The remodeling process may persist despite any further discrete myocardial injury and is accelerated in the face of ongoing risk factors such as hypertension, diabetes mellitus, cigarette smoking and elevated cholesterol.
In the developed world, CAD, either alone or in combination with hypertension, appears to be the dominant cause of HF.1,57 It is, however, difficult to definitively determine the primary etiology in a patient with multiple risk factors. Earlier studies which relied on non-invasive methods may have been unable to precisely determine the etiology (CAD in particular). In the Bromley Heart Failure Study, the percentage with unknown cause declined from 42% to 10% after cardiac catheterization and nuclear perfusion scanning.57 Variations in the frequencies of causes of HF can also be explained by differences in the study population and in the methods used to allocate HF diagnosis and underlying precipitant.
Coronary artery disease and myocardial infarction
In the original Framingham cohort, monitored until 1965, hypertension appeared to be the most common cause of HF (in 30% of men and 20% of women, and as co-factor in a further 33% and 25% respectively). However, in subsequent years, CAD emerged as the dominant etiology (increasing from 22% in the 1950s to almost 70% in the 1970s).58 Similarly, in the Glasgow MONICA Study, 95% of symptomatic individuals had evidence of CAD.15 Those with symptomatic HF were also more likely to have a past history of MI and ongoing angina pectoris. In asymptomatic individuals, 71% had evidence of CAD. As such, preventive strategies for CAD are also likely to be effective in preventing HF.
There are surprisingly few data describing the epidemiology of HF following acute MI. This again reflects the heterogeneous characteristics of the population. Clinical trials may provide a comprehensive data source but are confounded by bias related to inclusion criteria. Multivariable analyses from the Argatroban in Acute Myocardial Infarction (ARGMI)-2 trial suggest that risk of post-MI HF increases with age, anterior and Q-wave infarcts and in those with prior history of MI.59 Those with significant LVSD were also more likely to have symptomatic HF on presentation and have associated co-morbid conditions. The Worcester Heart Attack Study (MA, USA) has systematically evaluated the community incidence of MI and subsequent HF in a stable population over a 26-year period (1975–2001).60 Over this period the incidence of first MI decreased although the proportion of HF complicating MI rose from 40.1% in the mid 1970s, peaking in the early 1980s at 45.5%. Since then, there has been a gradual reduction in the proportion of HF, with a rate of 39.9% in 2001. Whilst the risk of death from HF declined in a parallel fashion, the adjusted odds ratio (OR) in the 2001 cohort was significantly higher (OR 1.37, 95% CI 1.13–1.64 compared with 1975–8). This has been confirmed by the most recent analyses, reflecting an MI population that is typically older and more often burdened by co-morbid conditions, particularly diabetes, and thus reflecting the evolution of the epidemiology of HF as a whole.
Hypertension
The measurable contribution of hypertension appears to have declined; Kannel and colleagues demonstrated a decline in the contribution of hypertension per decade through 1950–87 of approximately 5% in men and 30% in women.61 This may reflect the parallel introduction of efficacious antihypertensives, as well as improved diagnostic discrimination in the assessment of suspected CAD (with the advent of coronary angiography). Nevertheless, whilst the risk of HF associated with hypertension is measurably smaller than that associated with CAD, hypertension contributes considerably to the epidemiology of HF as it occurs more frequently.59 Additionally, ECG evidence of left ventricular hypertrophy in the presence of hypertension carries an approximate 15-fold increased risk of HF.62
Diabetes mellitus
Dysglycemia is directly implicated in HF pathophysiology with accumulating evidence that diabetes is associated with accelerated myocardial fibrosis, hypertrophy and progressive dysfunction.63 Diabetes confers a twofold increased risk of HF, and this may be proportionately greater in younger individuals and in women.64 Those most at risk are generally older, with higher baseline HbA1 c and Body Mass Index (BMI), established diabetic microvascular complications, longer duration of disease and on insulin, and experiencing coronary disease.65–67
In an analysis of the Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) Study, 27% of patients had documented diabetes, 8% had undiagnosed diabetes, and a further 9% had abnormal fasting glucose levels.68 Further compeling evidence of the negative contribution of diabetes comes from the CHARM program. Diabetes emerged as one of the most powerful predictors of outcome, with an approximate doubling of risk of death or the composite outcome of cardiovascular death or HF hospitalization.69 Recent evidence indicates that elevated fasting glucose alone is an independent predictor of future HF hospitalization, suggesting that impaired glucose tolerance and insulin resistance is prognostically significant even without overt diabetes.70
Obesity and cardiometabolic risk factors
Cardiometabolic risk factors include: abdominal adiposity, hypertriglyceridemia, high total cholesterol, low HDL cholesterol, hypertension and fasting hyperglycemia. The relationship between lipid abnormalities and atherosclerosis is well established. Similarly, both elevated triglycerides and high ration of total to high-density lipoprotein (HDL) cholesterol have been found to be associated with increased risk of HF.71 In an analysis of the Framingham Heart Study, each incremental increase in BMI of 1 kg/m2 was associated with a 5% and 7% increased risk of HF in men and women respectively, with 11% of cases of HF in men and 14% in women being directly attributed to obesity.72 Similarly, the NHANES-I follow-up study suggested that obesity was associated with a 30% higher risk of incident HF.62
Tobacco smoking
Multivariable analyses from epidemiologic studies and clinical trial registries indicate that tobacco smoking is an independent risk factor for the future development of HF. A prior history of cigarette smoking was found in 42% of men and 24% of women who developed HF from the Framingham Study and approximately 17% of all incident cases could be attributed to cigarette smoking in the NHANES-I survey.18,62 There is both a direct and independent relationship between smoking and the development of ALVSD as well as a dose–response relationship strongly supporting causality.73
Special circumstances
Valvular heart disease
Although the measurable contribution of valvular heart disease has decreased (secondary to the decreased incidence of rheumatic heart disease in the developed nations), patients with HF frequently have valvular abnormalities. In the Glasgow MONICA Study, 25% of patients with symptomatic LVSD had abnormalities on echocardiography which may contribute to morbidity.15 Nevertheless, as discussed above, even in the developed world, atherosclerotic disease now dominates.
Other causes of cardiomyopathy
Long-term heavy alcohol consumption (>11 units per day) is a particularly important (and largely preventable) cause of dilated cardiomyopathy, especially in men in their late forties.74 An ever increasing list of candidate drugs (both therapeutic and recreational) is implicated in HF etiology.75
Special attention should be given to individuals receiving anthracycline and immunotherapy-based cancer treatment. In survivors of childhood cancer, the relative risk of HF is 15.1 times greater than siblings and is greatest with previous anthracycline-based chemotherapy or thoracic irradiation. Trastuzumab is a monoclonal antibody directed against the HER-2 receptor (expressed in around 25% of breast cancers). Although very effective in the management of these cancers (which are often more aggressive) trastuzumab has been linked with an increased risk of cardiomyopathy (prevalence of up to 11% in some studies).76
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree