Parameter
Chumbley et al. (1977) [35]
Lanham et al. (1984) [36]
Gaskin et al. (1991) [37]a
Comarmond et al. (2012) [39]
Abu-Shakra et al. (1994) [38]
Guillevin et al. (1999) [27]
Number of patients
30
16
21
383
12
96
Male/female sex ratio
21/9
12/4
14/7
199/184
6/6
45/51
Mean age (years)
47
38
46.5
50.3
48
48.2
Manifestation
Asthma
100
100
100
91
100
100
General symptoms
–
–
–
–
100
70
Lung infiltrates
27
72
43
38.6
58
38
Pleurisy
–
29
–
8.9
–
–
Ear, nose and throat
70
70
–
41.8
83
47
Mononeuritis multiplex
63
66
70
46
92
78
Gastrointestinal
17
59
58
23
8
33
Cardiac
16
47
15
27.4
42
30
Arthralgias
20
51
43
29.8
42
41
Myalgias
–
68
–
38.9
33
54
Skin
66
–
50
39.7
67
51
Purpura
–
48
–
22.5
–
31
Nodules
27
30
–
8.9
–
19
Kidney involvement
20
49
80
21.7
8
16
Initial Symptoms
CSS is most commonly revealed by the onset of vasculitis manifestations – mononeuritis multiplex, purpura and general symptoms – and eosinophilia, in a previously asthmatic patient. However, some patients may develop asthma or eosinophilia simultaneously with vasculitis and, albeit very rarely, in the weeks following its onset [27]. The clinical presentation of these ANCA-positive patients differs significantly from that of ANCA-negative patients, with more frequent mononeuritis multiplex and glomerular nephritis in the former, and more cardiomyopathy in the latter [7, 8]. Based on differences in the clinical symptoms observed in patients with and without ANCA, we suggested [8] that CSS might be divided into different clinical and pathophysiological subtypes, which could be managed better with more specifically adapted therapies.
General Symptoms
General symptoms, primarily fatigue, malaise, fever (58 %) and weight loss, are usually the first and foremost manifestations of this vasculitis. In a previously asthmatic patient, these symptoms, in conjunction with eosinophilia, should alert the clinician to a possible CSS flare.
Pulmonary Manifestations
Lung involvement, as noted above, is almost universal in CSS patients. Asthma (96–100 % of patients) [8, 27] most often precedes systemic vasculitis (mean interval: 8.9 ± 10.9 years) [27], but sometimes occurs simultaneously or later. The mean age of asthma onset in the future CSS patient is about 30 years. It is often severe, corticosteroid-dependent and associated with ear, nose and throat symptoms, including rhinitis (70 %), nasal obstruction, nasal polyposis and sinusitis (62.5 %) [27]; the latter is never destructive as in Wegener’s granulomatosis. Evidence shows that both rhinitis and asthma in CSS are less often allergy-related than among non-CSS asthmatic patients [6]. Typical lung radiographic findings are patchy and transient alveolar infiltrates. Alveolar hemorrhage, due to pulmonary small-vessel vasculitis is another, much less common, CSS manifestation (Fig. 8.1). It occurs predominantly among anti-MPO ANCA-positive patients [7, 8]. Dyspnea due to phrenic palsy has sometimes been described. Finally, pleural manifestations exist but are rare. CSS pleural effusions are often asymptomatic and are mainly characterized by eosinophil-rich exudates. Pleural vasculitis is rarely observed, representing only 2/96 (2.1 %) of our previously reported patients [27]. Mediastinal adenopathy is sometimes seen, usually when cardiomyopathy is present.
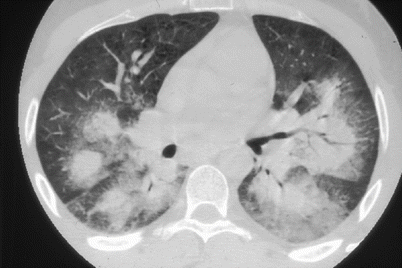

Fig. 8.1
Thoracic computed-tomography scan showing bilateral lung haemorrhages
Neurological Manifestations
Peripheral neuropathies are the second most common extrapulmonary manifestations of CSS, mainly mononeuritis multiplex, due to peripheral nerve vasculitis of the vasa nervorum. The most frequently involved nerves are the common peroneal (66 % of patients) [27], ulnar, internal popliteal and, to a lesser degree, radial and median nerves. Signs and symptoms are those of mononeuritis: motor palsy, sensory deficiency (hypo- and sometimes painful hyperesthesia) and muscular atrophy. Recovery is long and unpredictable. Cranial nerve palsies are much rarer (1 %). Polyneuropathy and Guillain–Barré-like syndromes have been described [40].
Central nervous system involvement is much less frequent. Clinical manifestations vary widely, ranging from hemiplegia to seizures, and rare subarachnoid hemorrhages. Computed-tomography (CT) scan efficiently diagnoses ischemic or hemorrhagic complications, but cannot attribute those manifestations to cerebral vasculitis. Magnetic resonance imaging (MRI) can detect T2-weighted signals in the subcortical white matter, which are highly evocative of vasculitis.
Skin Manifestations
Cutaneous involvement is diverse and frequent (40–70 %) in CSS patients. Two different mechanisms account for that diversity of skin lesions: vasculitis and extravascular granuloma. Vascular purpura was described in about half of published cases and 31.2 % of our patients [27], and predominantly affects the lower limbs. Other vasculitic manifestations include livedo reticularis (6.2 %), urticaria-like lesions (8.3 %) [27], Raynaud’s syndrome, vesicles and infiltrated papules. Subcutaneous nodules containing extravascular granulomas are present in about one-third of CSS patients. Clinically, they are red or violaceous, sometimes painful, nodules, preferentially located on the extensor surfaces of the elbow, fingers and scalp.
Gastrointestinal Involvement
Digestive tract involvement of CSS carries a poor prognosis [34, 41]. All types of histological lesions may be found: extravascular granulomas, which sometimes mimic polypoid lesions; tissue eosinophilia, presenting as eosinophilic enteritis and potentially affecting the entire gastrointestinal tract; and, finally, vasculitis, which is responsible for ulcerative ischemic lesions. The latter are the most severe, because they can be the origin of potentially fatal complications of intestinal hemorrhage and bowel perforation. Abdominal pain is a common CSS symptom, present in 30–60 % of the patients, along with nausea, vomiting and diarrhea. Sometimes, no intestinal lesions are found and abdominal pain may recede with treatment.
Heart Manifestations
Cardiac involvement is also responsible for a dismal prognosis, as it is the major cause of morbidity in CSS patients and the first cause of their mortality (48 % of deaths) [27]. Its frequency varies among studies, ranging from 15 to 84.6 %. Notably, 35 % of Sablé-Fourtassou et al. 112 CSS patients had cardiac manifestations [8]: 25 % with pericarditis and 24 % with myocarditis. Cardiac involvement was much more frequent in ANCA-negative than -positive patients (49 % vs. 12 %, respectively) [8], and 22.4 % vs. 5.7 %, according to Sinico et al. [7], suggesting that heart manifestations might mostly be due to mechanisms other than coronary vasculitis, e.g., myocardial granulomas or eosinophil toxicity. These manifestations include eosinophilic myocarditis, but also coronary vasculitis, heart valvulopathy [42], congestive heart failure, hypertension and pericarditis.
Complementary cardiac investigations are mandatory to evaluate clinically symptomatic patients and useful to detect cardiac involvement in asymptomatic patients, as non-negligible numbers of them have anomalies. These investigations comprise chest X-ray, electrocardiogram, echocardiography, and N-terminal pro-brain natriuretic peptide and troponin I dosages. Cardiac MRI (CMRI) is crucial to confirming CSS cardiac involvement, revealing inflammatory pericardial involvement, microvasculitis of the epicardial and myocardial vessels, and inflammation and/or fibrosis of the endocardial or myocardial tissue [43, 44]. One of our recent prospective studies showed that, while CMRI usually confirmed all cases of suspected (symptomatic) heart involvement (9/9 cases), it could also detect cardiac anomalies (visualized as delayed gadolinium enhancement of the myocardium) in almost 40 % of asymptomatic patients (4/11 patients) but the meaning of those findings remains uncertain [44, 45]. Comparing positron-emission tomography (PET) to CMRI showed that the former could detect active disease, whereas the latter is not always able to differentiate among activity, healing processes and sequelae [46].
Rheumatological Manifestations
These symptoms include arthralgias, usually without arthritis, and myalgias: 53 % of our patients had polyarthralgias, predominantly affecting the large joints, whose evolution often paralleled that of the vasculitis, but no joint deformation was observed. Myalgias are present in 53.6–68.7 % [8, 27] of CSS patients, especially during the acute phase of vasculitis.
Renal Manifestations
Kidney involvement is usually rare, compared to other ANCA-associated vasculitides. Only 16 % of our patients had renal involvement, consisting of merely mild proteinuria, hematuria and/or arterial hypertension [27]. However, because rapid crescentic glomerulonephritis (Fig. 8.2) causes higher mortality, making an early diagnosis is crucial. Serum creatinine levels exceeding 140 μmol/l (150 in the revisited version of the five-factor score (FFS) [34]) is another poor-prognosis factor [34]. Glomerular nephritis in CSS is usually associated with anti-MPO ANCA-positivity [7, 8]. However, other histological findings may be encountered: renal vasculitis, eosinophil-rich interstitial infiltrates and granulomas.
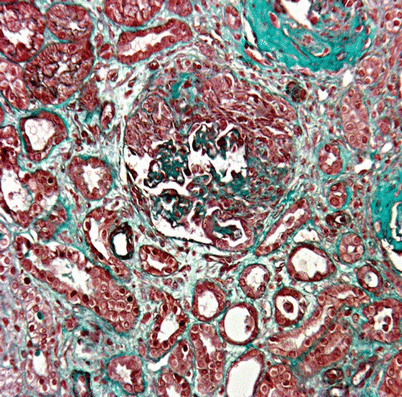

Fig. 8.2
Renal biopsy showing segmental necrotizing glomerulonephritis with crescents and interstitial inflammatory cell infiltrate. Masson’s trichrome stain, ×200
Miscellaneous
Eye involvement is rare in CSS. Ocular manifestations include: episcleritis, conjunctival nodules (consisting of extravascular granulomas), keratitis, posterior uveitis [47], ocular palsies and ischemic optic neuropathies.
Other particular organ locations have been described, for example, ureteral granulomas and stenosis, widespread digestive involvement [48, 49] or temporal arteritis mimicking giant-cell arteritis [50].
Finally, some apparently limited forms of CSS have been described [51, 52]. They concern patients with asthma, hypereosinophilia and single-organ involvement without systemic symptoms. Most often, the gut is involved as eosinophilic enteritis. In forms initially limited to the lung, the differential diagnosis with chronic eosinophilic pneumonia may be particularly difficult [53]. These two entities sometimes overlap, as cases of CSS preceded by chronic eosinophilic pneumonia have been described [54]. The existence of limited forms of CSS remains controversial, especially when clinical symptoms are associated only with eosinophilia, and their diagnosis is probably overestimated.
Complementary Investigations
Blood hypereosinophilia, high IgE titers and anti-MPO P-ANCA–positivity are the 3 main laboratory anomalies found in CSS. Inflammation is present in 80 % of these patients; it is intense and often accompanied by anemia (83 %) [27].
Eosinophilia fluctuates during CSS but is a constant symptom. An eosinophil count exceeding 1,500/mm3 or 10 % of the total white blood cell count has been retained as one of the diagnostic criteria for CSS [36]. We compared the accuracy of the absolute number of eosinophils vs the percentage to diagnose CSS and found them comparable [55], with mean values of the former ranging from 4,400 to 8,190 [7, 8, 27]. But eosinophils may disappear rapidly once corticosteroids are started. Eosinophilia is a relatively reliable marker of CSS activity, as an eosinophil rise may precede relapse. However, during follow-up of our patients, we frequently observed isolated eosinophil increases, usually between 1,000 and 1,500/mm3, although sometimes more, which remain difficult to interpret in the absence of clinical symptoms. Usually, this phenomenon occurs during steroid tapering; in that case, eosinophil values normalize when the steroid dose is again raised, sometimes by as little as 1 or 2 mg/day. Such isolated eosinophilia warrants careful monitoring but should not be considered sufficient to diagnose a CSS relapse. It is probably a predictive marker of relapse or a limited form of relapse, since recrudescence of asthma and eosinophilia preceded relapses with systemic manifestations in half of our patients (Guillevin, unpublished observations).
IgE is elevated at diagnosis in 75 % of patients but is non-specific, and is usually not seen in patients taking corticosteroids for their asthma. That observation further questions the role of allergy in CSS pathophysiology and explains why omalizumab, an anti-IgE monoclonal antibody, may sometimes be used to treat CSS.
ANCA, predominantly P-ANCA of anti-MPO specificity, are present in ~40 % of CSS patients but some can be directed against proteinase 3 (PR3). ANCA titers do not correlate with disease-evolution characteristics, although the authors of 1 study [56] favored a link between high ANCA titer and relapse. Unfortunately, that study included mainly microscopic polyangiitis and Wegener’s granulomatosis patients; furthermore, recruitment was based solely on laboratory immunological findings, which might have skewed interpretation of data.
Rheumatoid factor-positivity was reported for 22 (53.7 %) of the 41 published cases [36, 57]. Antinuclear antibodies are usually absent.
Bronchoalveolar lavage fluid can contain eosinophils [58]. Renal involvement should be sought by measuring the serum creatinine level and urinalysis, to search for proteinuria and hematuria. Finally, biopsies with histological evidence of granulomas (18 %), tissue eosinophilia (52 %) and/or necrotizing small-vessel vasculitis (55 %) also contribute to diagnosing CSS [8].
Among imaging techniques, chest X-ray should be the first examination. In our experience, it revealed abnormalities in 37.5 % of the patients [27], who had bilateral and migratory infiltrates or mixed interstitial patchy alveolar opacities, which can be further evaluated by thoracic CT scans.
Abdominal angiography, when performed, may show typical stenoses consistent with vasculitis in up to one-third of the patients [27], extremely rarely associated with microaneurysms. It may be informative for suspected mesenteric vasculitis, when other diagnostic methods fail. Angiography (conventional, CT scan or MRI) is not recommended before renal biopsy because CSS renal manifestations (glomerulonephritis) are usually associated with ANCA and involvement of larger vessels has not been described.
The systematic detection of cardiac abnormalities is important because of their poor prognosis. Coronary arteriography may be decisive in detecting underlying ischemic cardiopathy, distinct from CSS cardiomyopathy. Recently, CMRI was shown to be better at detecting myocardial involvement in CSS patients [44–46]. After gadolinium injection, T1-weighted cardiac sequences may reveal centromyocardial, subepicardial and/or subendocardial myocardial delayed enhancement. While CMRI always confirms symptomatic myocardiopathy, it may also help identify asymptomatic cardiac abnormalities that go undetected by other techniques. However, whether these patients will indeed develop patent cardiomyopathy and, thus, be at greater risk of death, has not yet been elucidated., Notably, unlike CMRI, cardiac PET scans are able to visualize the activity of lesions [46].
Diagnosis
Today, CSS diagnosis is essentially based on clinical manifestations and histology. The onset of vasculitis symptoms, such as mononeuritis multiplex or purpura in conjunction with systemic symptoms of fatigue, weight loss and fever, in a previously asthmatic patient are highly suggestive of CSS. Eosinophilia and anti-MPO ANCA further corroborate the diagnosis. Finally, although not mandatory, histological proof of CSS can confirm the diagnosis, with skin, nerve and muscle biopsy sites having the highest respective sensitivities of 67.4, 65.7, and 47.9 % [27]. For example, biopsies confirmed CSS for 91.6 % of our patients [27].
Histological Findings
Initially, CSS diagnosis of was mainly based on histological findings, characterized by the 3 known defining pathological lesions: small- to medium-sized–vessel vasculitis; eosinophil infiltration of the arterial wall and adjacent tissues; and, finally, extravascular granuloma. During CSS, virtually any organ can be affected. During the acute phase of this vasculitis, arterial wall inflammation is characterized by fibrinoid necrosis of the media, and pleomorphic cellular infiltrates, predominantly eosinophils, around and inside the arterial wall. This lesion slowly progresses towards complete vessel fibrosis, resulting in the obliteration of its lumen (Fig. 8.3). Extravascular granulomas, although quite characteristic of CSS, are neither constant in nor specific to CSS [59, 60], as they can be seen in other vasculitides, such as Wegener’s granulomatosis, or autoimmune diseases. Finding all 3 lesions on a single biopsy is very rare, and less than a fifth of the patients’ specimens will satisfy all 3 pathological criteria simultaneously [61]. This observation has led to decreased reliance on histological features alone, as they are considered too stringent for diagnosing CSS.
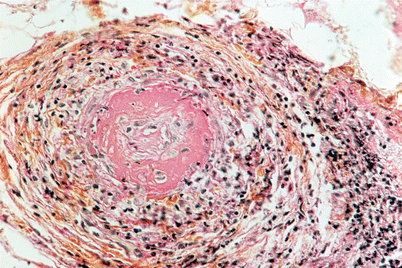

Fig. 8.3
Deep skin biopsy showing intraluminal thrombus, fibrinoïd necrosis and intense interstitial inflammatory cell infiltrate containing eosinophils. Hematoxylin, eosin and saffron stain, ×400
Diagnostic Criteria
A clinical redefinition of CSS, established in 1984 by Lanham et al. [36], has allowed clinicians diagnose CSS with good specificity and sensitivity without relying on histological findings. The 3 diagnostic criteria are asthma, blood eosinophilia exceeding 1,500/mm3, and evidence of vasculitis involving 2 or more organs. However, that definition has been criticized: first, asthma may follow and not precede the vasculitic phase; second, eosinophilia may sometimes fluctuate and disappear, either spontaneously or after starting corticosteroids; and, finally, vasculitis, despite certain characteristic clinical manifestations, may be hard to confirm without a biopsy.
Other criteria have been proposed, especially for classification purposes, notably the American College of Rheumatology [62] and Chapel Hill criteria [2]. However, those criteria are not diagnostic, as their goal is to classify patients as having a probable diagnosis of CSS, but only once vasculitis is already the presumptive diagnosis. New diagnostic criteria currently being discussed include, for example, anti-MPO ANCA, and clinical and biological redefinitions of CSS.
Box 8.1
Eosinophilic Granulomatosis with Polyangiitis: Diagnostic Criteria
At present, no validated diagnostic criteria for Churg Strauss syndrome (CSS) exist. In 1956, Churg and Strauss described pathological features of their eponymic syndrome. In 1984, Lanham et al were the first to propose clinicopathological criteria. To date, both in clinical practice and scientific publications, the American College of Rheumatology and Chapell Hill classification criteria are the most commonly used. In the absence of documented evidence of vasculitis, these classification criteria may be unable to differentiate between CSS and other hypereosinophilic syndromes.
Lanham (or Hammersmith Hospital) 1984 Criteria
1.
asthma
2.
peak peripheral blood eosinophil count >1,500/mm3
3.
systemic vasculitis involving ≥2 extrapulmonary organs
When all three criteria are met: diagnostic sensitivity and specificity are both 95 %.
American College of Rheumatology 1990 Criteria
1.
asthma
2.
eosinophilia >10 % of the differential white blood cell count
3.
mono- or polyneuropathy attributable to systemic vasculitis
4.
non-fixed pulmonary infiltrates
5.
paranasal sinus abnormality (history of acute or chronic paranasal sinus pain or tenderness, or radiographic opacification of the paranasal sinuses)
6.
extravascular eosinophils on biopsy
When at least four of these six criteria are met, diagnostic sensitivity and specificity are 85 and 99.7 %, respectively.
The Chapel Hill 1994 Consensus Conference Definition of CSS
1.
asthma
2.
eosinophilia
3.
eosinophil-rich and granulomatous inflammation involving the respiratory tract
4.
necrotizing vasculitis affecting small- to medium-sized vessels
Differential Diagnosis
The differential diagnosis of CSS depends on each patient’s predominant clinical manifestations. Before vasculitis onset, concomitant eosinophilia, asthma and lung infiltrates may resemble certain parasitic infections, like helminthiases, or allergic bronchopulmonary aspergillosis. At this stage, it may be difficult to differentiate chronic eosinophilic pneumonia from CSS [53]. However, when systemic vasculitis manifestations appear, asthma and a somewhat lower sensitivity to corticosteroids tend to favor a diagnosis of CSS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


