Evidence is accumulating that toll-like receptors (TLR) are involved in the initiation and progression of cardiovascular disease. Enhanced expression of these receptors on monocytes has been shown in patients with acute coronary syndrome (ACS). However, expression on platelets in this group of patients has not been evaluated yet. We aimed to demonstrate the possible relationship of platelet TLR-2 and TLR-4 expressions with stable coronary artery disease and ACS pathogenesis. In this observational case–control study, 40 patients diagnosed with ACS (unstable angina pectoris, non–ST-segment elevation and ST-segment elevation ACS), 40 patients diagnosed with stable coronary artery disease, and 40 age- and gender-matched subjects with normal coronary arteries were involved. Platelet TLR-2 and TLR-4 expressions were evaluated by flow cytometry in peripheral venous blood samples obtained before coronary angiography. A total of 120 patients (60.7 ± 12.3 years, 50% men) were included. Median platelet TLR-2 and TLR-4 expressions were greater in patients with ACS compared to those with stable angina pectoris and normal coronary arteries (29.5% vs 10.5% vs 3.0%, p <0.001 and 40.5% vs 11.5% vs 3.0%, p <0.001, respectively). Median platelet TLR-2 and TLR-4 expressions were also greater in patients with stable angina pectoris compared to those with normal coronary arteries (p <0.05). In conclusion, this is the first study demonstrating enhanced TLR-2 and TLR-4 expressions on platelets in patients with ACS. These findings may suggest that platelet TLR expression as a novel potential prophylactic and therapeutic target in ACS.
Besides their primary role in hemostasis, platelets have been shown to secrete several adhesion molecules that make them essential in the interplay between endothelial cells and other immune cells. Platelets have also been shown to interact and modulate leukocyte function through the release of mediators such as P-selectin and CD40-L or formation of platelet–leukocyte aggregates. Toll-like receptor (TLR) expression on platelets may be the link between platelets and immune response that deserves attention. Previous studies have primarily focused on the role of TLR expression on platelets mostly concerning the pathogenesis of thrombocytopenia, sepsis, hemorrhagic shock, or severe thrombotic complications (such as stroke or myocardial infarction) on the basis of infections. However, TLRs have been reported to sense not only microbial molecules but also molecules of host origin. This has been suggestive of the possible role of TLRs on atherosclerosis, which is regarded as an inflammatory disease. In this study, we aimed to demonstrate the possible association between platelet TLR-2 and TLR-4 expressions with stable coronary artery disease (CAD) and acute coronary syndrome (ACS).
Methods
Forty patients diagnosed with ACS (unstable angina pectoris [USAP], non–ST-segment elevation myocardial infarction [NSTEMI], ST-segment elevation myocardial infarction [STEMI]), and 40 patients with stable angina pectoris were included as the patient group, where 40 age-and gender-matched patients with normal coronary arteries were included as the control group. Patients were recruited between November 2013 and June 2014.
In our study, ACS group was defined as the group of patients who were diagnosed with ACS in accordance with the most recent guidelines. Other patients were recruited from the outpatient clinics of our department. These patients had presented with stable angina pectoris and were scheduled for invasive coronary angiography after clinical evaluation and assessment of ischemia in accordance with the recommendations of guidelines on the management of stable angina pectoris. Patients with normal epicardial coronary arteries in the coronary angiography were classified as the “normal coronary arteries” group. Patients who had significant CAD in the coronary angiography were classified as “stable coronary artery disease” group.
Detailed medical history was recorded from each patient to identify co-morbidities, cardiovascular risk factors, and drug use. All patients underwent detailed physical examination. Transthoracic echocardiographic examination was performed to evaluate left ventricular systolic functions.
Patients with a history of coronary artery disease, renal dysfunction (glomerular filtration rate <60 ml/min/1.73 m 2 calculated with Modification of Diet in Renal Disease Study equation), malignancy, or previous surgery within the past 3 months were not included in the study. None of the patients had any evidence of systemic inflammatory disease (e.g., infections, autoimmune diseases). In addition, none had received antiplatelet or anticoagulant drugs before. The study was performed in accordance with the Declaration of Helsinki and was approved by the local Research Ethics Committee. All participants provided written informed consent.
Peripheral venous blood samples were collected from all patients at the first encounter (i.e., the emergency room for patients with ACS and outpatient clinic visits for stable angina pectoris and control groups). Complete blood cell count, serum biochemistry tests including fasting blood glucose, renal function tests were performed in addition to creatine kinase-myocardial band and troponin- T measurements and flow cytometry.
Flow cytometry was performed within 15 minutes of blood sample obtainment. Whole blood (5 ml) collected in citrate was centrifuged for 15 minutes at 210 g , and the platelet-rich plasma fraction was collected. Platelets were washed twice with buffer solution (17.5 mM Na 2 HPO 4 , 8.9 mM Na 2 EDTA, 154 mM NaCl, pH 6.9, containing 0.1% bovine serum albumin) by centrifuging them 5 minutes at 2.310 g . Platelets were re- suspended in HEPES medium (132 mM NaCl, 6 mM KCl, 1 mM MgSO 4 , 1.2 mM KH 2 PO 4 , 20 mM HEPES, pH 7.4, containing 5 mM glucose). TLR expression on platelets was measured by flow cytometry (Beckman Coulter XL-MCL Flow Cytometer, CA). Briefly, 10 6 platelets were incubated with 10 μl TLR-2 PE (CD282, clone T2.5, Biolegend) and 10 μL TLR-4 FITC (CD284, HTA 125, Biolegend) antibodies, and isotopic controls (MsIgG1 FITC, Biolegend and MsIgG1 PE, Biolegend) for 30 minutes at 4°C in the dark and were washed once with phosphate-buffered saline (PBS); then 10.000 events were acquired through a live gate drawn on forward light scatter and side light scatter.
Normally distributed continuous parameters are presented as mean ± standard deviation, and skewed continuous parameters are expressed as median (interquartile range defined as the difference between twenty-fifth and seventy-fifth centiles). Categorical data are presented as frequencies and percentages. Comparisons of continuous data among the 3 patient groups with normal distribution were performed using analysis of variance followed by the Bonferroni correction. Skewed data from 3 patient groups were compared with the Kruskal–Wallis H test followed by the adjusted Mann–Whitney U test. Comparison of 2 groups was made with the Mann–Whitney U test and the Student t – test for parameters with and without normal distribution, respectively. Multinomial logistic regression analysis was performed to determine whether platelet TLR expression was independently associated with ACS occurrence. Spearman correlation analysis was done to investigate the factors related with levels of platelet TLR-2 and TLR-4 expressions. Statistical analyses are performed using SPSS statistical software version 20.0 (SPSS Inc., Chicago, Illinois). A 2-tailed p <0.05 is considered statistically significant.
Results
A total of 120 patients (60.7 ± 12.3 years; 50.0% men) were included in the study. Patients were evaluated in separate groups, each containing age- and gender-matched 40 patients, depending on their clinical presentation. Baseline characteristics of the patients regarding their clinical presentation are provided in Table 1 . Of the cardiovascular risk factors, prevalence of hypertension (p = 0.015), diabetes mellitus (p = 0.013), smoking (p = 0.023), and family history of CAD (p = 0.006) were higher in patients with ACS compared to patients with stable angina pectoris and normal coronary arteries. Patients with stable angina pectoris and ACS had higher prevalence of hyperlipidemia compared to patients with normal coronary arteries (p = 0.013). In contrast, body mass index did not differ between 3 groups (p = 0.595). Patients with stable angina pectoris more frequently received β blockers (p = 0.001) and statins (p <0.001), where it was the group of patients with ACS who more frequently received angiotensin-converting enzyme inhibitor/angiotensin receptor blockers (p = 0.001; Table 1 ).
| Variable | Total population (n= 120) | Normal coronary arteries (n= 40) | Stable coronary artery disease (n= 40) | Acute coronary syndrome (n= 40) | p value |
|---|---|---|---|---|---|
| Age (years) | 60.7± 12.3 | 60.4± 12.1 | 60.5± 12.2 | 61.0± 12.6 | 0.712 |
| Men | 60 (50.0%) | 19 (47.5%) | 20 (50.0%) | 21 (52.5%) | 0.789 |
| Conventional risk factors for coronary artery disease | |||||
| Hypertension | 64 (53.3%) | 15 (37.5%) | 21 (52.5%) | 28 (70.0%) | 0.015 ∗ |
| Diabetes mellitus | 48 (40.0%) | 10 (25.0%) | 15 (37.5%) | 23 (57.5%) | 0.013 ∗ |
| Hyperlipidemia | 82 (68.3%) | 20 (50.0%) | 31 (77.5%) | 31 (77.5%) | 0.013 ∗ |
| Smoking | 16 (13.3%) | 2 (5.0%) | 4 (10.0%) | 10 (25.0%) | 0.023 ∗ |
| Family history of coronary artery disease | 25 (20.8%) | 5 (12.5%) | 5 (12.5%) | 15 (37.5%) | 0.006 ∗ |
| Body mass index (kg/ m 2 ) | 28.5± 3.4 | 28.1± 3.2 | 29.5± 3.3 | 28.3± 3.1 | 0.595 |
| Medications on admission | |||||
| Statin | 75 (62.5%) | 13 (32.5%) | 36 (90.0%) | 26 (65.0%) | <0.001 ∗ |
| Beta blocker | 68 (56.7%) | 13 (32.5%) | 28 (70.0%) | 27 (67.5%) | 0.001 ∗ |
| Angiotensin- converting enzyme inhibitor/ Angiotensin receptor blocker | 65 (54.2%) | 15 (37.5%) | 21 (52.5%) | 29 (72.5%) | 0.001 ∗ |
| Laboratory parameters | |||||
| Serum creatinine (mg/ dL) | 0.9± 0.2 | 0.8± 0.2 | 0.9± 0.2 | 0.9± 0.3 | 0.689 |
| Fasting blood glucose (mg/dL) | 101.5 (37.0) | 91 (25.5) | 99 (33.0) | 119 (47.0) † ‡ | 0.001 ∗ |
| Low- density lipoprotein cholesterol (mg/dL) | 130.7± 37.4 | 143.7± 41.5 | 126.2± 32.3 | 124.0± 36.6 | 0.078 |
| High- density lipoprotein cholesterol (mg/dL) | 43.2± 13.5 | 50.5± 14.8 | 48.3± 13.2 | 35.1± 7.9 † ‡ | <0.001 ∗ |
| Hemoglobin (g/dL) | 13.8± 1.5 | 13.5± 1.6 | 13.7± 1.3 | 14.1± 1.6 | 0.246 |
| White blood cell count (x10 3 /μL) | 8.2± 3.1 | 7.1± 0.2 | 7.9± 2.9 | 9.5± 3.9 † ‡ | 0.002 ∗ |
| Platelet count (x10 3 /μL) | 242.6± 65.6 | 253.2± 68.7 | 233.1± 64.0 | 241.8± 64.4 | 0.417 |
| Creatine kinase- myocardial band (ng/mL) § | |||||
| Baseline | – | – | – | 2.4 (2.9) | – |
| Peak | – | – | – | 6.9 (18.2) | |
| Troponin- T (ng/mL) § | |||||
| Baseline | – | – | – | 0.03 (0.11) | |
| Peak | – | – | – | 0.41 (2.3) | – |
| Echocardiographic parameters | |||||
| Left ventricular ejection fraction (%) | 56.9± 10.5 | 60.0± 10.4 | 59.1± 9.1 | 52.3± 10.5 † ‡ | 0.003 ∗ |
† Denotes the statistical significant difference when compared to the group of patients with normal coronary arteries.
‡ Denotes the statistical significant difference when compared to the group of patients with stable angina pectoris.
White blood cell (WBC) count was significantly greater in patients with ACS (p = 0.002). Fasting blood glucose levels were higher in patients with ACS compared to others (p = 0.001). High-density lipoprotein (HDL) cholesterol levels were significantly lower in patients with ACS (p <0.001), in which low-density lipoprotein (LDL) cholesterol (p = 0.078) and serum creatinine (p = 0.689) levels did not significantly differ between 3 groups ( Table 1 ).
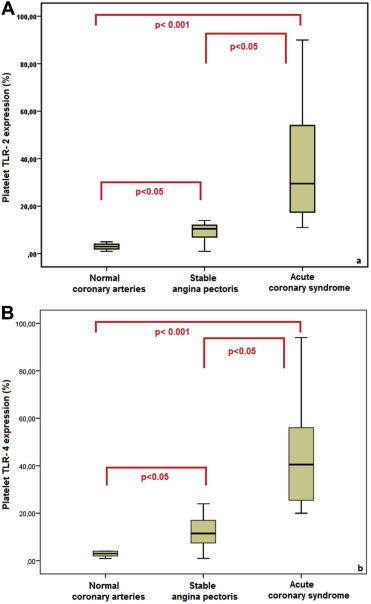
Median platelet TLR-2 and TLR-4 expressions were greater in patients with ACS compared to those with stable angina pectoris and normal coronary arteries (29.5% vs 10.5% vs 3.0%, p <0.001 and 40.5% vs 11.5% vs 3.0%, p <0.001, respectively). Median platelet TLR-2 and TLR-4 expressions were also greater in patients with stable angina pectoris compared to those with normal coronary arteries (p <0.05; Figure 1 ). Platelet TLR-2 (p = 0.799) or TLR-4 (p = 0.715) expressions on platelets in patients with USAP, NSTEMI, and STEMI did not differ among groups ( Table 2 ).
| Platelet Toll- like receptor expression (%) | Unstable angina pectoris (n=12) | Non-ST- segment elevation myocardial infarction (n=16) | ST- segment elevation myocardial infarction (n=12) | p value |
|---|---|---|---|---|
| Toll- like receptor- 2 | 26.0 (47.0) | 29.5 (44.0) | 32.0 (74.0) | 0.799 |
| Toll- like receptor- 4 | 29.5 (37.0) | 45.5 (74.0) | 51.5 (70.0) | 0.715 |
Median platelet TLR-2 and TLR-4 expressions were found to be greater in patients with hypertension (p = 0.021 and p = 0.012), diabetes mellitus (p = 0.009 and p = 0.002), hyperlipidemia (p = 0.020 and p = 0.047), smoking (p = 0.014 and p = 0.048), and family history of CAD (p = 0.028 and p = 0.020; Table 3 ).
| Variables | Platelet Toll- like receptor- 2 expression | p value | Platelet Toll- like receptor−4 expression | p value | |
|---|---|---|---|---|---|
| Hypertension | – | 6.5 (9.5) | 0.021 ∗ | 5.5 (16.3) | 0.012 ∗ |
| + | 13 (19.8) | 19.0 (29.8) | |||
| Diabetes mellitus | – | 6.0 (9.0) | 0.009 ∗ | 5.5 (17.0) | 0.002 ∗ |
| + | 13.5 (22.5) | 20.0 (36.3) | |||
| Hyperlipidemia | – | 5 (11.0) | 0.020 ∗ | 4.0 (21.0) | 0.047 ∗ |
| + | 12 (20.0) | 13.5 (24.5) | |||
| Smoking | – | 8.0 (10.0) | 0.014 ∗ | 10.5 (18.3) | 0.048 ∗ |
| + | 16.5 (36.3) | 20.0 (31.8) | |||
| Family history of coronary artery disease | – | 8.0 (10.0) | 0.028 ∗ | 8.0 (17.5) | 0.020 ∗ |
| + | 14.0 (43.0) | 24.0 (39.0) | |||
| Beta blockers | – | 5 (11.0) | 0.011 ∗ | 4.0 (20.5) | 0.006 ∗ |
| + | 12 (12.0) | 15 (28.0) | |||
| Statins | – | 4.0 (14.8) | 0.009 ∗ | 4.0 (22.8) | 0.027 ∗ |
| + | 12.0 (12.0) | 15.0 (21.0) | |||
| Angiotensin- converting enzyme inhibitor/ angiotensin receptor blockers | – | 5.0 (11.0) | 0.008 ∗ | 4.0 (21.0) | 0.005 ∗ |
| + | 12.0 (19.0) | 17.0 (29.0) |
Results of correlation analysis between platelet TLR-2 and TLR-4 expressions and baseline characteristics are provided in Table 4 . Fasting blood glucose, WBC count, peak troponin- T were positively and HDL cholesterol and left ventricular ejection fraction were inversely correlated with platelet TLR-2 expression. Fasting blood glucose, LDL cholesterol, WBC count, peak creatine kinase-myocardial band, baseline troponin- T, and peak troponin- T were positively and HDL-cholesterol and left ventricular ejection fraction were inversely correlated with platelet TLR-4 expression ( Table 4 ).
| Variables | Platelet Toll- like receptor −2 expression | Platelet Toll- like receptor −4 expression | ||
|---|---|---|---|---|
| Spearman’s rho | p value | Spearman’s rho | p value | |
| Body mass index (kg/m 2 ) | -0.300 | 0.121 | -0.469 | 0.125 |
| Fasting blood glucose (mg/ dL) | 0.353 | 0.001 ∗ | 0.386 | <0.001 ∗ |
| Low- density lipoprotein cholesterol (mg/dL) | -0.135 | 0.190 | -0.265 | 0.175 |
| High- density lipoprotein cholesterol (mg/dL) | -0.496 | <0.001 ∗ | -0.556 | <0.001 ∗ |
| Left ventricular ejection fraction (%) | -0.329 | 0.001 ∗ | -0.379 | <0.001 ∗ |
| Hemoglobin (g/dL) | 0.126 | 0.179 | 0.128 | 0.174 |
| White blood cell count (x10 3 /μL) | 0.272 | 0.003 ∗ | 0.304 | 0.001 ∗ |
| Platelet count (x10 3 /μL) | -0.073 | 0.443 | -0.076 | 0.423 |
| Baseline creatine kinase- myocardial band (ng/mL) | 0.081 | 0.554 | 0.112 | 0.413 |
| Peak creatine kinase- myocardial band (ng/mL) | 0.168 | 0.215 | 0.328 | 0.014 ∗ |
| Baseline troponin- T (ng/mL) | 0.186 | 0.170 | 0.318 | 0.017 ∗ |
| Peak troponin- T (ng/Ml) | 0.282 | 0.035 ∗ | 0.452 | <0.001 ∗ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


