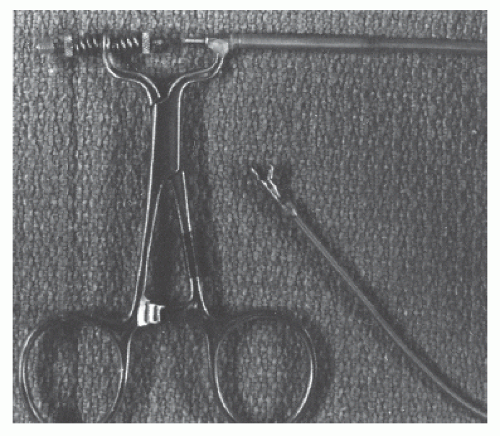
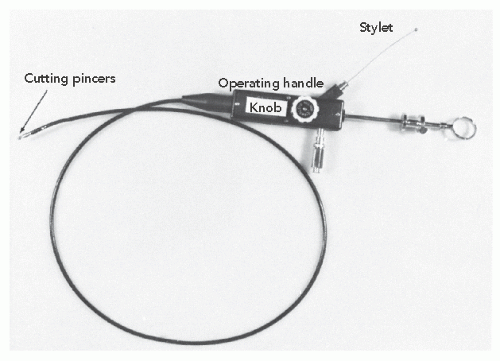
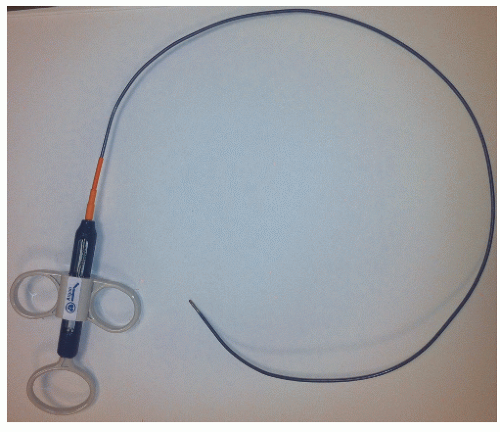
cutting stylet or cutting needle and the elongated specimen obtained was then sectioned for appropriate examination. Nearly all (192 of 198) of these patients had tissue recovered, with half of them showing nonspecific hypertrophy and interstitial fibrosis, 13% small vessel disease, and the rest showing nonspecific basophilic degeneration, amyloidosis, rheumatic heart disease, or myocarditis. The validity of the percutaneous biopsy was confirmed in 11 patients who later died, allowing full postmortem examination of the heart. Complications of this technique included pericardial tamponade in eight and postpericardiotomy syndrome in an additional four patients.
Table 26.1 Equipment for Endomyocardial Biopsy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
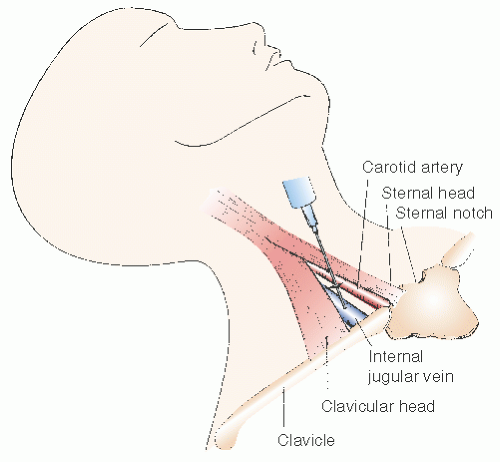
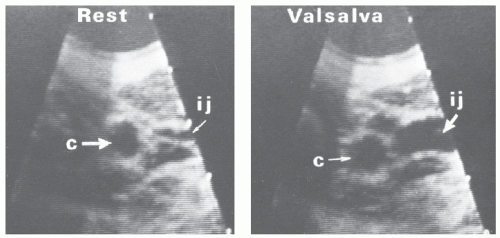
occurs during the procedure. In addition, this higher location in the anterior triangle lessens the risk of pneumothorax. If the location of the internal jugular vein is not readily apparent, we routinely evaluate the neck vasculature echocardiographically, using a 7.5-MHz dedicated sector scanner (Site Rite, Dynamax Corporation, Pittsburgh, PA) or a conventional echo transducer encased in a sterile sheath. The jugular vein can be distinguished from the more medial carotid artery by location, the pulsatility of the artery, and the compressibility of the vein (Figure 26.5). Use of echo guidance has been demonstrated to increase the frequency of successful vein cannulation, decrease access time, and decrease complication rates.14,15
a no. 11 surgical blade. The incision is then expanded with the tip of a mosquito clamp to ensure that the skin will accommodate the 7F venous sheath. In a classic approach, the 22-gauge anesthesia needle is directed toward the anticipated venous pathway at an angle of approximately 30° to 40° from vertical and 20° right of the sagittal plane and is advanced in small increments, aspirating before infiltration of small amounts of lidocaine to provide local anesthesia. Excess lidocaine infiltration should be avoided, since it may result in venous compression or infiltration of vocal cords or carotid sheath resulting in transient hoarseness or Horner syndrome.
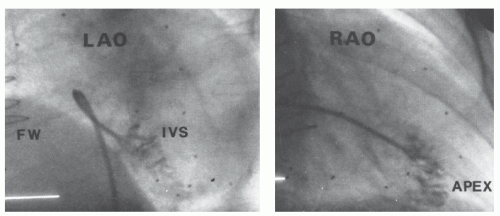
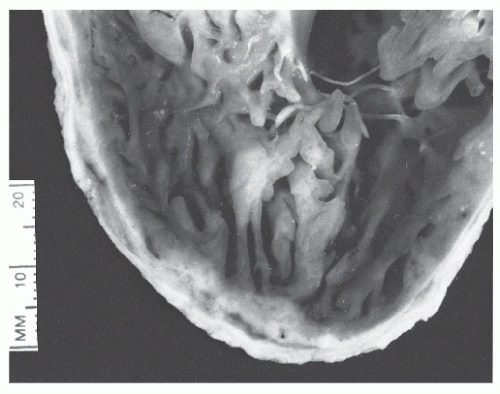 Figure 26.8 Postmortem specimen shows heavy trabeculation of the interior surface of the right ventricle and the thinness of the right ventricular free wall. |
monitor and right ventricular pressure demonstrated, or a gentle contrast injection can be performed. The tip of the preformed sheath should be free floating rather than positioned against the ventricular myocardium or trabeculated portion of the right ventricle muscle. Once in a stable position, the sheath should be aspirated and flushed with heparinized solution. The sheath should be connected to a constant-infusion port to maintain patency and avoid clot formation.
injury to the right ventricular free wall, which is only 1 to 2 mm thick. Patients with pulmonary hypertension, a bleeding diathesis, or right ventricular enlargement may be at increased risk for right ventricular perforation. Any patient complaining of sharp pain during the performance of the endomyocardial biopsy should be considered to have experienced cardiac perforation. Patients in whom perforation occurs immediately complain of a visceral pain and within 1 to 2 minutes may develop bradycardia and hypotension. This is in part owing to an exaggerated vagal response, but limited benefit is achieved by atropine administration. No further biopsy attempts should be made until the significance of the patient’s complaints has been fully investigated. This may include fluoroscopy of the heart border, measurement of the right atrial pressure waveform, or performance of a portable echocardiogram. Patients with suspected perforation should have their right atrial pressure and the pulsatility of the right and left heart borders continuously monitored. Loss of pulsation of heart borders and increased right atrial pressure are strong indicators for pericardial tamponade. Echocardiography should be obtained immediately to determine the presence and severity of pericardial blood accumulation, and it is our preferred method for assessing and monitoring patients with suspected perforation. Cardiovascular collapse or electrical-mechanical disassociation in the setting of a biopsy should be considered to be presumptive evidence of pericardial tamponade, and the operator must be prepared to do an immediate pericardiocentesis, even before echocardiographic confirmation of tamponade.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree