Endocrinology & the Heart
Eveline Oestreicher Stock, MD
Endocrinology involves the study of glands that secrete hormones into the circulation for effects at distant target sites. Classic endocrine glands include organs like the pituitary gland, thyroid and parathyroid glands, pancreatic islets, adrenal glands, ovaries, and testes. It is now clear that hormones are also secreted from nontraditional endocrine organs and play critical roles in the regulation of physiologic processes. Examples of such organs include the heart (natriuretic peptides), kidney (renin and erythropoietin), adipose tissue (leptin, adiponectin, and irisin), and gut (cholecystokinin and incretins). Once in the circulation, hormones bind to receptors on target tissues to elicit biological effects. Target tissues for some hormones (eg, glucocorticoid, thyroid hormone) can be numerous, reflecting the wide distribution of receptors, while other tissues may have a more limited distribution (eg, androgens). Because hormone receptors can be so ubiquitous throughout the body, the presence or absence of a single hormone can have multiple effects on one or more organ systems, including the cardiovascular system. This chapter considers most of the common and some uncommon endocrinopathies that can affect the heart, addressing specifically how they can be recognized and treated to best restore cardiovascular health.
THYROID & THE HEART
The cardiovascular signs and symptoms of thyroid disease are some of the most characteristic and clinically relevant signs and symptoms seen. Both hyperthyroidism and hypothyroidism produce changes in cardiac contractility, myocardial oxygen consumption, cardiac output, blood pressure, and systemic vascular resistance. Although it is well known that hyperthyroidism can produce atrial fibrillation, it is less well recognized that hypothyroidism predisposes to ventricular dysrhythmias. The importance of the recognition of the effects of thyroid disease on the heart is highlighted by the recognition that restoration of normal thyroid function in almost all cases reverses the abnormal cardiovascular changes.
Thyroid disease is quite common, affecting approximately 9–15% of the adult female population and a smaller percentage of males. This gender-specific prevalence likely results from autoimmune causes for the most common forms of thyroid disease, such as Graves and Hashimoto disease. However, with advancing age, especially beyond the eighth decade of life, the incidence of disease in males increases to equal that of females.
Thyroid hormone regulates oxidative and metabolic processes throughout the body by directing cellular protein synthesis at the nuclear level. Nongenomic actions of thyroid hormones have also been recognized based on rapid tissue responses that take place before RNA transcription could occur and by recognition of triiodothyronine (T3) and thyroxine (T4) binding sites outside of the nucleus. Both overproduction and underproduction of thyroid hormone can disrupt normal metabolic function. Under the control of pituitary release of thyroid-stimulating hormone (TSH), the thyroid gland secretes T4 and T3, mostly bound to plasma proteins. The free, or unbound, fraction of hormone negatively feeds back at the level of the hypothalamus and pituitary to suppress further release of thyroid-releasing hormone (TRH) and TSH.
 Cardiovascular Effects of Thyroid Hormones
Cardiovascular Effects of Thyroid Hormones
The mechanism of thyroid hormone–induced dysfunction is multifactorial. Thyroid hormones increase the number of β-adrenergic receptors in the heart and skeletal muscle, adipose tissue, and lymphocytes. They also amplify catecholamine action at a postreceptor site. The heart rate increases due to increased sinoatrial activity, a lower threshold for atrial activity, and shortened atrial repolarization. These last two factors also create a favorable substrate for the generation of atrial fibrillation (AF), and a similar effect on ventricular myocardium has been associated with ventricular arrhythmias. In addition, vascular volume increases due to activation of the renin–angiotensin system, and there is increased contractility due to increased metabolic demand and the direct effect of T3 on cardiac muscle. Systemic vascular resistance decreases because of T3-induced peripheral vasodilation. The sum of these effects is a dramatic increase in cardiac output, frequently to more than 7 L/min.
Many of the clinical manifestations of thyrotoxicosis appear to reflect increased sensitivity to catecholamines. Therapy with β-adrenergic blocking agents is often helpful in controlling these sympathomimetic manifestations of thyroid hormone excess.
In addition, thyroid hormone has numerous effects on coagulation, such as shortened activated partial thromboplastin time, increased fibrinogen levels, and increased factor VIII and factor X activity, and clinical sequelae such as stroke are seen in patients with thyrotoxicosis, even in sinus rhythm. Although undocumented paroxysmal AF may contribute to embolic phenomena, studies suggest that hyperthyroidism is associated with a prothrombotic state and ischemic stroke independent of atrial arrhythmias.
1. Hyperthyroidism
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Low (suppressed) thyroid-stimulating hormone levels (below the lower range).
Low (suppressed) thyroid-stimulating hormone levels (below the lower range).
![]() High free T4, total T4, and free thyroxine index, and/or high free T3 or total T3 radioimmunoassay.
High free T4, total T4, and free thyroxine index, and/or high free T3 or total T3 radioimmunoassay.
![]() High 24-hour radioactive iodine uptake in Graves disease or toxic multinodular goiter; low uptake in thyroiditis or exogenous cause.
High 24-hour radioactive iodine uptake in Graves disease or toxic multinodular goiter; low uptake in thyroiditis or exogenous cause.
![]() Goiter (often with bruit) and exophthalmos in Graves disease.
Goiter (often with bruit) and exophthalmos in Graves disease.
 General Considerations
General Considerations
In general, thyrotoxicosis is the clinical syndrome that results when tissues are exposed to high levels of circulating thyroid hormones. Short-term hyperthyroidism is characterized by a high cardiac output state with an increase in heart rate and cardiac preload and a reduction in peripheral vascular resistance, resulting in a hyperdynamic circulation. Cardiac preload (left ventricular end-diastolic volume) is increased as a consequence of the increase in blood volume and the enhancement of diastolic function. The reduction in systemic vascular resistance is responsible for decreased renal perfusion pressure and subsequent activation of the renin–angiotensin–aldosterone system, resulting in increased sodium absorption and blood volume. In experimental studies, thyroid hormone induced physiologic cardiomyocyte hypertrophy by acting on intracellular signaling pathways. In humans, long-term exposure to thyroid hormone excess may exert unfavorable effects on cardiac structure and function because it may increase left ventricular mass, arterial stiffness, and left atrial size and may induce diastolic dysfunction, thereby impairing left ventricle performance. However, because thyroid hormone excess does not induce cardiac fibrosis, these changes are reversed once euthyroidism is restored.
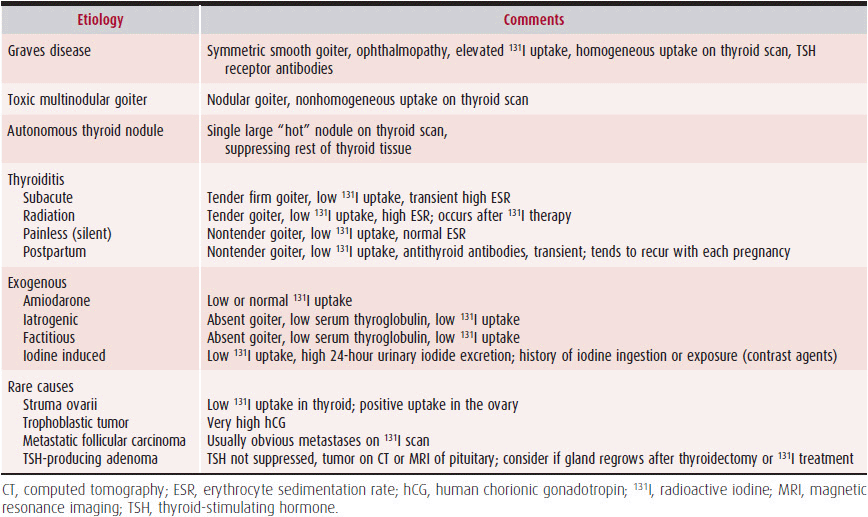
In most instances, thyrotoxicosis is due to hyperactivity of the thyroid gland, or hyperthyroidism. Occasionally, thyrotoxicosis may be due to other causes such as excessive ingestion of thyroid hormone. The various forms of hyperthyroidism are listed in Table 34–1.
Table 34–1. Causes of Hyperthyroidism
 Clinical Findings
Clinical Findings
A. History
A family history of thyroid disease should be investigated, including a history of goiter, as well as immunologic disorders such as type 1 diabetes, rheumatoid disease, pernicious anemia, vitiligo, or myasthenia gravis, which may be associated with an increased incidence of autoimmune thyroid disease. Iodine ingestion in the form of amiodarone, an iodine-containing antiarrhythmic drug, or intravenous iodide-containing contrast media used in angiography and computed tomography (CT) scanning may induce hyper- or hypothyroidism. Lithium carbonate, used in the treatment of bipolar disorder, can also induce hypothyroidism, goiter, and more rarely, hyperthyroidism. Residence in an area of low dietary iodide is associated with iodine deficiency goiter (endemic goiter). Exposure to ionizing radiation in childhood has been associated with increased incidence of thyroid disease.
B. Symptoms & Signs
Patients with hyperthyroidism often complain of weight loss despite an increased appetite; this helps distinguish this condition from other wasting conditions such as cancer or acquired immunodeficiency syndrome (AIDS) where appetite is usually diminished. A fine resting tremor of the hands is noticed, along with nervousness, anxiety, insomnia, mood swings, and irritability. Heat intolerance and diaphoresis are seen. Proximal muscle weakness and muscle wasting may be prominent. An increased number of bowel movements or diarrhea may occur due to accelerated transit in the gut. Thyroid enlargement, thyrotoxic eye signs (eg, lid retraction, proptosis, periorbital edema, conjunctival redness, extra-ocular muscle involvement) and tachycardia are commonly seen. In patients over age 60, cardiovascular and myopathic manifestations predominate; the most common presenting complaints are palpitations, dyspnea on exertion, tremor, nervousness, and weight loss.
Patients with overt and subclinical hyperthyroidism are at increased risk of cardiac death, although the exact mechanism leading to this effect is not well established. The increased risk of cardiac mortality might be a consequence of the increased risk of atrial arrhythmias and of the risk of heart failure (HF) in these individuals, especially in elderly patients. In particular, thyrotoxic AF has been associated with an increased risk of cerebrovascular and pulmonary embolism. Furthermore, autoimmune hyperthyroidism has been associated with autoimmune cardiovascular involvement: pulmonary arterial hypertension, myxomatous cardiac valve disease, and autoimmune cardiomyopathy have been reported in patients with Graves disease with higher frequency than in the general population.
AF is the most common cardiac complication of hyper-thyroidism, occurring in an estimated 10–25% of overtly hyperthyroid patients compared with 0.4% of the general population. High-normal thyroid levels or subclinical hyperthyroidism is also associated with an increased risk of developing AF. The prevalence of AF in both populations increases with age; other risk factors for AF in thyrotoxic patients include male sex, valvular heart disease, ischemic heart disease, and congestive HF. In the elderly, AF may be the only manifestation of thyrotoxicosis, a condition known as apathetic hyperthyroidism. Other atrial dysrhythmias, such as paroxysmal atrial tachycardia and atrial flutter, are unusual. Ventricular arrhythmias usually indicate underlying cardiac disease.
The overall incidence of arterial embolism or stroke in thyrotoxic AF appears to be increased, but the reported range varies widely in clinical trials (8–40%). The recommendations for thromboembolic prophylaxis are controversial. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines state that, although the topic is controversial and increased risk has not been definitively proven, anticoagulation treatment is recommended “in the absence of a specific contraindication, at least until a euthyroid state has been restored and heart failure has been cured.” It should be noted that the effect of vitamin K antagonists is affected by the patient’s thyroid status—elevated thyroid levels increase the anticoagulation effect, and antithyroid agents (such as propylthiouracil or methimazole) may diminish the effect. Careful monitoring of anticoagulation levels is required during the treatment of patients with AF and hyperthyroidism. Given the lack of clear evidence, the ACC/AHA classification of thyrotoxicosis as a moderate thromboembolic risk factor appears reasonable, and the recommendation to initiate anticoagulation when there are no contraindications appears to be warranted. More evidence-based trials are needed to clarify this issue.
Other cardiac manifestations of hyperthyroidism are symptomatic HF (which occurs in approximately 6% of patients) and pulmonary arterial hypertension. Less than 1% of patients with hyperthyroidism develop dilated cardiomyopathy with left ventricular systolic dysfunction. Hyperthyroid patients most commonly complain of exercise intolerance and exertional dyspnea, which are largely explained by inadequate increase in cardiac output during exercise. Impaired exercise tolerance in these patients is a sign that the hyperthyroid heart cannot further accommodate the increase in cardiovascular demand during physical exercise. Changes in loading conditions, loss of sinus rhythm, or a reduction in myocardial contractility may further impair the efficiency of the cardiovascular system in hyperthyroid patients, thereby inducing congestive HF. The development of orthopnea, paroxysmal nocturnal dyspnea, peripheral edema, and neck vein distension may indicate the progression to advanced HF. However, the clinical manifestations and degree of HF in hyperthyroid patients depend on a variety of factors, including the patient’s age, the cause and severity of hyperthyroidism, and the underlying cardiac conditions. Often, thyrotoxicosis precipitates exacerbation of angina when the increased demands placed on the heart by the thyrotoxic state are accompanied by the underlying fixed atherosclerotic lesions of coronary artery disease. The angina improves once the thyrotoxicosis is treated, and frank myocar-dial infarction precipitated by thyrotoxicosis is rare.
In most conditions, a goiter is present. Absence of a goiter, especially in a young person, should raise the suspicion of factitious hyperthyroidism; elderly patients, however, may not have a palpable goiter in the presence of disease.
The precordium is hyperdynamic, and loud heart sounds and systolic ejection murmurs may be heard, reflecting increased cardiac flow across the valves. The pulse is rapid and bounding. The skin has an unusually soft and velvety texture and is often sweaty. There is proximal muscle weakness, with patients often having difficulty rising from a squatting position. Deep tendon reflexes are hyperreflexic, and a resting tremor is present. Dermopathy or localized edema may be present on the shins (pretibial myxedema, also known as peau d’orange).
In younger patients, especially young women, Graves disease is the most common cause of thyrotoxicosis. Graves disease is an autoimmune disease in which antibodies to the TSH receptor stimulate both excessive thyroid growth and thyroid hormone production. The disease may occur at any age, with a peak incidence in the 20- to 40-year age group. These patients typically have a symmetric goiter (often with a bruit) with or without exophthalmos. Toxic multinodular goiter is a more common diagnosis in patients over the age of 40. Usually these goiters are large and nodular (as the name suggests). Iatrogenic or factitious thyrotoxicosis should always be considered; these patients typically have no goiter, and the thyroglobulin level is suppressed. Clinicians should suspect hyperthyroidism in patients with persistent sinus tachycardia and AF, unexplained congestive HF, or unstable angina.
C. Diagnostic Studies
1. Electrocardiography & echocardiography—Sinus tachycardia is usually present, although any supraventricular tachycardia can be seen. AF occurs in 10–20% of hyperthyroid patients; its prevalence in the population at large is 0.4%. On echocardiography, a hypercontractile state is seen with rapid filling of a highly compliant ventricle. Increased left ventricular mass and cardiac hypertrophy can also be seen.
2. Laboratory findings—Diagnosis is made by measurement of thyroid function tests (Table 34–2). TSH should be suppressed below the lower limit of detection, and the free T4 or free thyroxine index (FTI) should be elevated, confirming the diagnosis. If the free T4 or FTI is normal, measurement of total or free T3 is recommended to rule out a condition known as T3 thyrotoxicosis, in which the serum T4 level is normal but the total or free T3 is elevated. If the only abnormality is a suppressed TSH level, subclinical hyperthyroidism versus a systemic nonthyroidal illness must be considered. Thyroid function tests should be repeated after any period of illness to determine whether the abnormal thyroid function tests have resolved and were due to nonthyroidal illness.
Table 34–2. Tests in Hyperthyroidism
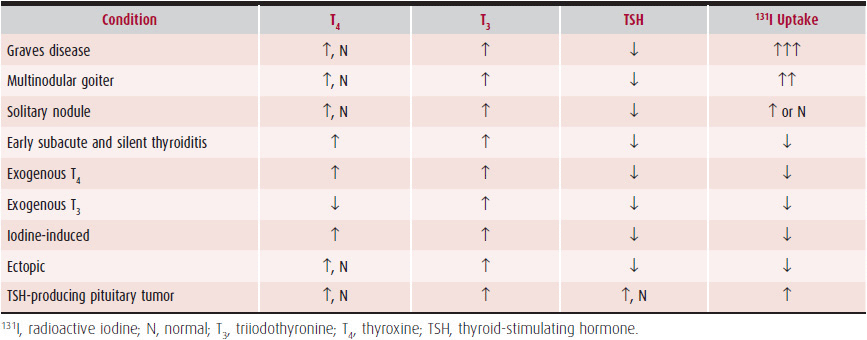
Although only about one-third of patients have eye involvement clinically, enlarged muscles can be detected by imaging in over 90% of patients. If eye signs are present, the diagnosis of Graves disease can be made without further tests. If eye signs are absent and the patient is hyperthyroid (with or without goiter), a radioactive iodine uptake (RAIU) test should be done. Elevated RAIU is seen in Graves disease, toxic multinodular goiter, and occasionally, an autonomously functioning thyroid nodule. In contrast, a decreased RAIU is seen in thyroiditis and exogenous hyperthyroidism. A low RAIU is also found in patients who are iodine-loaded or who are taking T4 therapy. Thyroid scans rarely add any useful information to a good physical examination in patients with diffusely enlarged thyroid glands. In Graves disease, the scan typically shows an enlarged symmetric gland with homogeneous uptake. Thyroid scans are occasionally helpful in identifying an adenoma or multinodular gland, in which one or more cold spots are seen. Other tests that may be helpful, include measurement of antithyroid antibodies (antimicrosomal or thyroid peroxidase antibodies) or TSH receptor antibody (TSAb), which are relatively specific for patients with Graves disease. Thyroglobulin levels will be suppressed in patients with factitious or iatrogenic thyrotoxicosis.
 Treatment
Treatment
Treatment is directed at rapidly improving symptoms and reducing demands on the heart. The mainstay of treatment is accomplished by preventing thyroid hormone synthesis and release with antithyroid drugs, followed by radioactive iodine thyroid ablation (Table 34–3); surgery may also be indicated. β-Blockers are most commonly used to improve symptoms. If the tachycardia is considered to be significantly deleterious in patients with HF, esmolol, which has a rapid onset of action and short half-life, may be given intravenously; it should be stopped—with rapid reversal—if HF worsens. Tremor and tachycardia will improve almost immediately with β-blocker therapy, although systolic and diastolic contractile performance will not change due to direct effects of thyroid hormone on cardiac muscle. Of the oral β-blockers, propranolol is preferred because it also prevents the peripheral conversion of T4 to T3. The dose should be titrated to the patient’s pulse and is usually 20–80 mg four times daily. Occasionally, high doses (100–320 mg four times daily) of propranolol are required in thyroid storm.
Table 34–3. Agents Used to Treat Hyperthyroidism
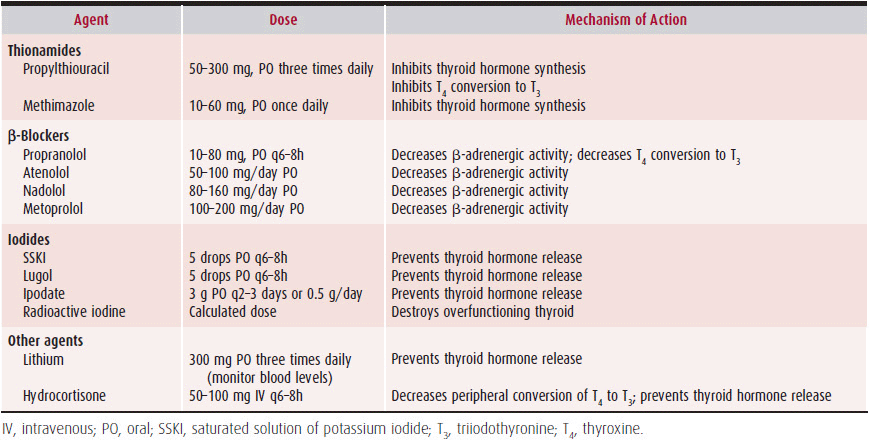
Thionamides are used to prevent thyroid hormone release and synthesis, by blocking iodine oxidation, organification, and iodotyrosine coupling. Propylthiouracil (PTU) and methimazole are the thionamides used in the United States. Because they deplete intrathyroidal stores of thyroid hormone, they circumvent the precipitation of thyroid storm that can result from radiation thyroiditis after radioactive iodine ablation. Doses typically begin at 50–100 mg three times daily of PTU and 10–30 mg daily of methimazole. Methimazole may be preferred because of its once-a-day dosing and lower incidence of side effects, such as the potential for severe hepatotoxicity with PTU. PTU is preferred in pregnant women, in the first trimester, because of rare teratogenic effects of methimazole. These drugs are typically withdrawn 3–5 days prior to radioactive iodine ablation and restarted 3–5 days after ablation. Thionamides are known to cause nausea and rash (in about 5% of patients) and, of most concern, agranulocytosis (in about 0.5% of patients). Agranulocytosis is often heralded by severe sore throat and fever and requires immediate cessation of antithyroid drug therapy. Thus, all patients receiving antithyroid drugs are instructed to stop the drug and contact their physician to obtain a complete blood count if sore throat or fever develops during treatment.
Other drugs, including lithium, iodides, and corticosteroids, are usually reserved for the prevention of life-threatening conditions such as thyroid storm; occasionally, they are used for patients with severe congestive HF or unstable angina secondary to thyrotoxicosis. Lithium prevents thyroid hormone release, and the dosage is determined by monitoring therapeutic serum levels. Iodides abruptly prevent the release of thyroid hormone. They must be used in conjunction with thionamides because rebound or escape occurs commonly. Doses are usually 3–5 drops of a supersaturated potassium iodide solution or Lugol solution (50 mg of iodide per drop) every 6–8 hours.
Parenteral corticosteroids are usually given in stress doses for thyroid storm. Corticosteroids inhibit thyroid hormone secretion and prevent peripheral conversion of T4 to T3. Doses are usually 50–100 mg of hydrocortisone every 6–8 hours.
Radioactive iodine (131I) is the preferred and definitive treatment for thyrotoxicosis in patients with a high RAIU. Because thyroid tissue is the only tissue that requires iodine (for thyroid hormone synthesis), 131I is used for thyroid gland destruction. The advantages of radioactive iodine include the fact that usually only a single treatment is needed and that it is relatively safe and inexpensive. Because the treatment usually requires 3–6 months to resolve the hyperthyroidism, most patients will require interim therapy with thionamides during that time. The patient is usually rendered hypothyroid as a result of the treatment and is then treated with long-term thyroid hormone replacement.
Patients with multinodular goiters, who have lower RAIU (than do patients with Graves disease), often have an inadequate response to 131I and may require re-treatment or surgery. Pregnant patients should not receive 131I and should, therefore, be treated with PTU, with or without β-blockers, or with subtotal thyroidectomy in the second trimester.
Aspirin, nonsteroidal anti-inflammatory drugs, and—rarely—corticosteroids are used if painful thyroiditis is present. Thyroiditis is reversible and requires short-term therapy only. β-Blockers can be used temporarily to improve thyrotoxic symptoms.
Treatment of congestive HF and AF is essentially the same as for a euthyroid individual. Treatment should include use of a nonselective β-blocker (eg, propranolol) or a selective β1-blocker (eg, metoprolol) to normalize the heart rate. The physician should be aware, however, that treatment of AF is limited to control of the ventricular rate because cardioversion will not be successful as long as the thyrotoxicosis is present. In addition, patients may be relatively resistant to digoxin. Usually, sinus rhythm returns within 6 weeks with resolution of the thyrotoxic state. Older patients with underlying cardiac disease may not spontaneously revert and may require cardioversion. Anticoagulation should be considered until the patient is euthyroid and in sinus rhythm.
Once the patient becomes euthyroid, the hyperdynamic cardiovascular manifestations disappear. AF should convert to normal sinus rhythm in more than 60% of patients, and angina should improve because of decreased demands on the heart.
 Prognosis
Prognosis
The prognosis is generally excellent for most hyperthyroid conditions. Graves disease and autonomously functioning thyroid nodules usually respond well to 131I and do not recur. As noted previously, multinodular goiters may be relatively resistant to 131I and may ultimately require subtotal thyroidectomy. Despite surgical treatment, multinodular goiters frequently recur.
Frost L, et al. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. 2004; 164(15):1675–8. [PMID: 15302638]
Klein I, et al. Thyroid disease and the heart. Circulation. 2007; 116:1725–35. [PMID: 17923583]
Sheu JJ, et al. Hyperthyroidism and risk of ischemic stroke in young adults: a 5-year follow-up study. Stroke. 2010;41(5):961–6. [PMID: 20360542]
2. Hypothyroidism
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() TSH levels above normal range (primary hypothyroidism).
TSH levels above normal range (primary hypothyroidism).
![]() Low free T4 or low FTI.
Low free T4 or low FTI.
 General Considerations
General Considerations
Hypothyroidism is a clinical syndrome resulting from thyroid hormone deficiency, which results in a generalized slowing down of metabolic processes, with slowed heart rate, diminished oxygen consumption, and deposition of glycosaminoglycans in extracellular spaces, particularly in skin and muscle; in extreme cases, the clinical syndrome of myxedema occurs, which is associated with hypothermia, hypoventilation, hypotension, and central nervous system signs. Overt hypothyroidism affects ~3% of the adult female population; it is estimated that anywhere from 0.5% to 5.0% of the adult population of the United States has underlying hypothyroidism.
Hypothyroidism is associated with accelerated atherosclerosis, likely from the accompanying hyperlipidemia and diastolic hypertension seen in these patients. Hypothyroid patients have other atherosclerotic cardiovascular disease risk factors, such as increased C-reactive protein and homo-cysteine, and appear to have increased risk of stroke. The atherosclerosis is especially pronounced in the presence of hypertension; however, angina is uncommon, and the incidence of myocardial infarction is not increased. This is probably due to the decreased metabolic demands placed on the heart in the hypothyroid state. More commonly, angina is precipitated or worsened by rapid thyroid hormone replacement.
 Clinical Findings
Clinical Findings
A. Symptoms & Signs
1. Systemic symptoms & signs—Hypothyroidism is an insidious disease and may be subtle in its progression and presentation. Patients typically complain of weight gain (although morbid obesity does not occur), weakness, lethargy, fatigue, depression, muscle cramps, constipation, cold intolerance, dry skin, and coarse hair. Women often have menstrual disorders (most commonly, menorrhagia), and men may have impotence or decreased libido.
2. Cardiovascular symptoms & signs—Cardiovascular findings are the opposite of those found in hyperthyroidism. There is a decrease in cardiac output because of reduced ventricular contractility, bradycardia, increased peripheral resistance, and reduced blood volume. The hemodynamic alterations resemble those of congestive HF except that pulmonary congestion does not occur, and pulmonary artery and right ventricle pressures are often normal. In addition, cardiac output and systemic vascular resistance increase normally in response to exercise, unlike HF from other causes.
Cardiac enlargement may occur due to a combination of interstitial edema, left ventricular dilatation, and pericardial effusion. Myxedematous HF can be distinguished from other causes in that it responds to exercise with an increased heart rate; improves with thyroid hormone replacement, but not digitalis and diuretics; rarely results in pulmonary congestion; and exhibits high protein content effusions.
B. Physical Examination
Hypothermia, bradycardia with weak arterial pulses, and mild hypertension are characteristic vital signs. The hypertension may be due to increased peripheral resistance. Thyroid hormone replacement will normalize blood pressure in approximately one-third of these patients. The patient may appear pale, with periorbital edema and facial puffiness. Hair and skin are usually coarse and dry. Goiter is present in patients with Hashimoto thyroiditis, congenital enzyme deficiencies, iodine deficiency, and thyroid hormone resistance; it is also present in patients taking amiodarone and antithyroid drug therapy such as thionamides and lithium.
Percussion of the chest may reveal pleural effusions. Distant heart sounds are present, especially if a pericardial effusion is present. Reflexes are characteristically delayed in the return phase. Nonpitting edema may be present as a result of the deposition of mucopolysaccharides. Severe hypothyroidism can progress to myxedema coma, and anasarca may be present. In the presence of congestive HF, pitting edema may be superimposed on the nonpitting edema.
C. Diagnostic Studies
1. Electrocardiography & echocardiography—Electrocardiographic (ECG) changes include low-voltage QRS complexes and flattened or inverted T waves, sinus bradycardia, and prolonged PR and QT intervals. Prolonged QT may increase ventricular irritability and, rarely, induce torsade de pointes; this is reversible by treatment. Atrial, ventricular, and intraventricular conduction delays are three times as likely in patients with myxedema as in the general population. Pericardial effusion is probably partly responsible for these ECG changes.
Pericardial effusions occur in as many as 30% of all hypothyroid patients. Cardiac tamponade is unusual because of the slow accumulation of fluid, which does not increase pericardial pressure excessively.
2. Laboratory findings—Asymptomatic hypothyroid individuals, such as the elderly, frequently go unrecognized. By far, the most common cause of hypothyroidism in the United States is Hashimoto thyroiditis. Other causes of hypothyroidism are listed in Table 34–4.
Table 34–4. Causes of Hypothyroidism
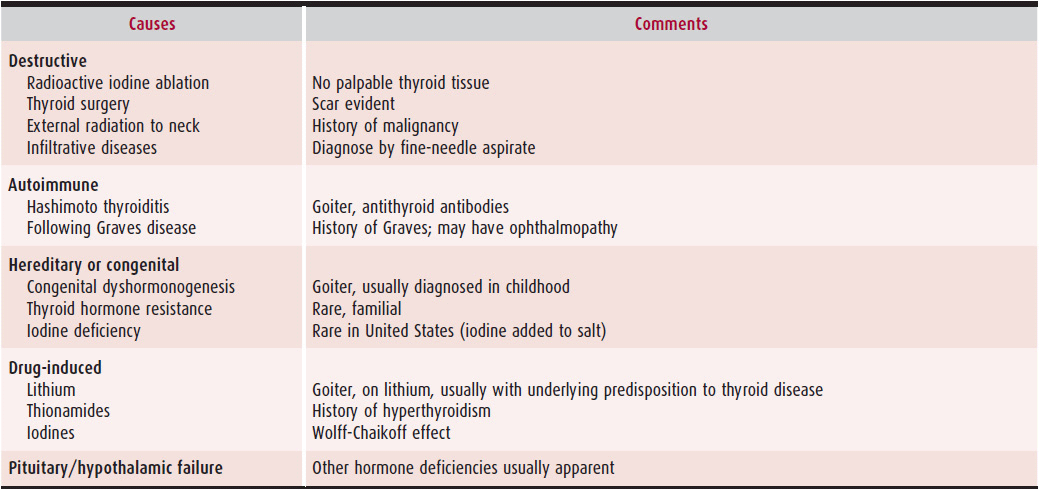
The combination of elevated serum TSH and low serum free T4 is diagnostic of primary hypothyroidism. Serum T3 levels are variable and may be within normal range. The absence of an elevated TSH level indicates either nonthyroidal illness or hypothalamic–pituitary dysfunction. Occasionally, TSH level is mildly elevated (usually < 10 mU/L) in the face of a normal T4 level. Subclinical hypothyroidism, as opposed to recovery from a nonthyroidal illness, must be considered. These patients are typically asymptomatic but are at intermediate risk for cardiovascular disease when compared with euthyroid or frankly hypothyroid individuals.
Antithyroid antibodies (antimicrosomal or thyroid peroxidase antibodies) are elevated in Hashimoto thyroiditis. Creatine kinase isoenzymes are increased in hypothyroidism; the isoenzyme pattern is usually MM and not MB. Hypothyroidism is a common cause of hyperlipidemia; 95% of hypothyroid individuals will have elevated low-density lipoprotein (LDL) cholesterol, and 70% will have elevation in both LDL cholesterol and triglycerides. Anemia of chronic disease may be seen, as well as hyponatremia from impaired free-water clearance.
 Treatment
Treatment
T4 therapy usually reverses the cardiovascular manifestations associated with hypothyroidism. Treatment with T4, which is available in pure form and is stable and inexpensive, is recommended. Because T4 is converted to T3 in peripheral tissues, both hormones become available once T4 is administered. Desiccated thyroid is now considered obsolete; it contains both T4 and T3 (as liothyronine). T3 is unsatisfactory because of its rapid absorption, short half-life, and transient effects. The half-life of T4 is 7 days, so it is given once daily. It is well absorbed, and blood levels are monitored following TSH levels. Replacement doses of T4 vary according to the patient’s age and body weight. Regarding cardiovascular effects of T4 supplementation, one of the most important considerations with thyroid hormone replacement therapy is the speed of rendering the patient euthyroid. Young patients without evidence of cardiac disease can be replaced with full doses of T4. Patients over the age of 55 or patients with evidence or suspicion of cardiac disease require slow and judicious use of thyroid hormone replacement to prevent exacerbation of angina or precipitation of myocardial infarction. The typical regimen would begin with 25 mcg (0.025 mg/day) or one-fourth of a normal replacement dose and increase the dose gradually after 6- to 8-week intervals based on serum TSH measurements; it may take several months to reach full replacement doses (100–150 mcg/day).
Patients with unstable angina and hypothyroidism may be specially challenging to treat because of the risk of exacerbating the angina. Very small doses of hormone should be used, and the dosage increments must be made slowly over a longer-than-usual time. If necessary, angioplasty or coronary artery bypass grafting (CABG) should be recommended, after which—if revascularization is complete—thyroid hormone replacement can occur at the usual dosage and rate. Such a strategy is likely to result in better surgical outcomes with improved morbidity and mortality. Adjustments in anesthesia and drug doses should be made because their decreased metabolic clearance makes hypothyroid patients very sensitive to these agents.
Treatment of myxedema coma is controversial. Many authors recommend high initial doses of intravenous T4 (400 mcg) to saturate receptors and replenish diminished stores, followed by 100 mcg/day. Others prefer a more conservative approach of 50–100 mcg/day intravenously. Stress doses of hydrocortisone should also be administered (100 mg intravenously every 6–8 hours) because hypothyroidism and adrenal insufficiency frequently coexist, and thyroid hormone replacement may precipitate adrenal crisis.
 Prognosis
Prognosis
In the absence of coexisting heart disease, treatment with thyroid hormone and restoration of a euthyroid status correct the hemodynamic, ECG, and serum enzyme alterations and restore heart size to normal. Therapy is lifelong, and relapses occur if the patient is noncompliant or taken off therapy for any reason.
Flynn RW, et al. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab. 2006;91(6):2159–64. [PMID: 16537678]
Hak AE, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: The Rotterdam Study. Ann Intern Med. 2000;132(4):270–8. [PMID: 10681281]
Monzani F, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab. 2001;86(3):1110–5. [PMID: 11238494]
Tielens ET, et al. Changes in cardiac function at rest before and after treatment in primary hypothyroidism. Am J Cardiol. 2000;85(3):376–80. [PMID: 11078310]
Toft AD, et al. Thyroid disease and the heart. Heart. 2000;84(4): 455–60. [PMID: 10995425]
3. Effect of Heart Disease on Thyroid Function
Acute or chronic illness, such as occurs with myocardial infarction, congestive HF, and during the postoperative period of cardiopulmonary bypass, can make the interpretation of thyroid function tests difficult. Levels of T3 and T4 can drop as much as 20–40%, the so-called euthyroid sick syndrome. TSH is inhibited centrally and can be suppressed further by use of drugs, such as dopamine or corticosteroids, to undetectable levels. As recovery from the underlying illness occurs, the TSH level may rise above normal into the hypothyroid range. Consequently, patients with significant cardiovascular disease in a coronary care unit are likely to have abnormal thyroid function tests. The interpretation of low serum thyroid hormones with acute or chronic illnesses should be done with great caution because of the importance of distinguishing between hypothyroidism and the euthyroid sick syndrome. There is no evidence to support thyroid hormone replacement in the latter patients, and it may be potentially harmful.
4. Cardiovascular Drugs & the Thyroid
Overall incidence of thyroid dysfunction in patients receiving amiodarone is estimated to be between 2% and 24%. Amiodarone-induced thyrotoxicosis (AIT) is more common in countries with low iodine uptake, and amiodarone-induced hypothyroidism is more common in areas that are iodine replete.
AIT may occur at any time during or even after amiodarone treatment, particularly in patients with an underlying predisposition to thyroid disease, such as those who have a goiter. Presenting symptoms and signs include weight loss, weakness, tremor, or new or recurrent atrial tachyarrhythmias. Classic symptoms may be masked by the anti-adrenergic effects of amiodarone. Biochemical diagnosis is straightforward if the T3 or free T3 level is elevated and the TSH level is suppressed to undetectable levels.
Pathogenesis is complex and can involve excessive thyroid hormone synthesis from the iodine load (so called type I AIT) or destructive thyroiditis (type II AIT). Features of both types of AIT are listed in Table 34–5.
Table 34–5. Features of Amiodarone-Induced Thyrotoxicosis
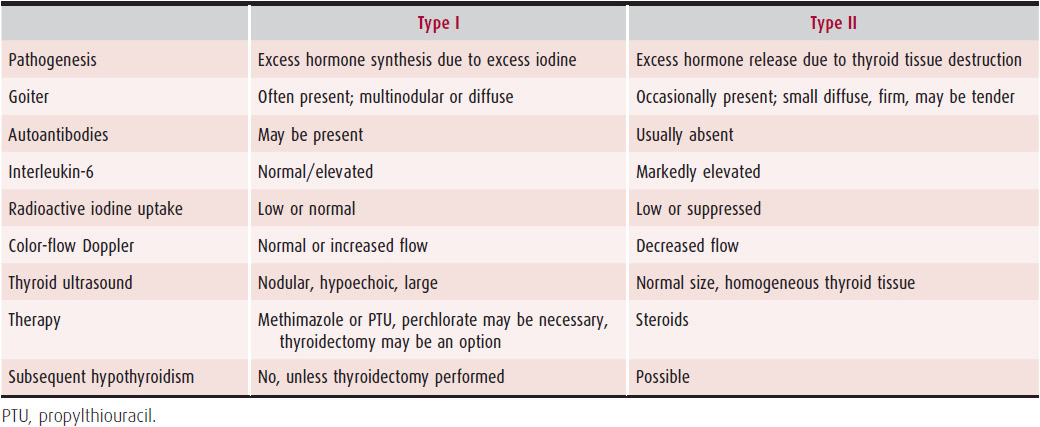
Therapy for AIT may be complex and requires knowledge of the underlying pathogenesis. In type I AIT, thionamides should be used to block further organification of iodine and synthesis of hormones. Larger than usual doses are often required (methimazole 40–60 mg/day or PTU 600–800 mg/day) because the iodine-rich gland is resistant to thionamide therapy. Potassium perchlorate (800–1000 mg/day for 15–45 days), a drug that inhibits iodine uptake into the gland, can also be used, although with caution because agranulocytosis, aplastic anemia, and nephrotic syndrome have occurred at doses > 1.5 g. Discontinuation of amiodarone may be recommended and necessary in some cases. Thyroidectomy can be undertaken in severe cases unresponsive to medical therapy.
Type II AIT can be treated with high-dose corticosteroids (prednisone 30–40 mg/day or equivalent) for 3 months with a gradual slow taper to minimize recurrence. Discontinuation of amiodarone is usually not necessary because the thyroiditis resolves within several weeks to months and rarely recurs.
If the two types of AIT cannot be distinguished, therapy with both corticosteroids and antithyroid drugs should be started.
Like amiodarone, radiologic contrast material containing iodine, such as that used in cardiac catheterizations, has the potential for causing transient thyrotoxicosis.
Daniels GH. Amiodarone induced thyrotoxicosis. J Clin Endocrinol Metab. 2001;86(1):3–8. [PMID: 11231968]
Martino E, et al. The effects of amiodarone on the thyroid. Endocrinol Rev. 2001;22(2):240–54. [PMID: 11294826]
PARATHYROID & THE HEART
Parathyroid hormone (PTH) is mainly responsible for the regulation of ionized calcium levels by concerted effects on three main target organs: bone, intestinal mucosa, and kidney. It participates in the regulation of calcium, phosphate, and magnesium homeostasis throughout the body. Although PTH itself has few effects on the heart, an excess or deficiency of this hormone can affect the cardiovascular system indirectly through its regulation of calcium.
1. Hyperparathyroidism
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Inappropriately normal or elevated PTH levels.
Inappropriately normal or elevated PTH levels.
![]() Serum calcium level above upper limit of normal (> 10 mg/dL) corrected for serum albumin, or ionized calcium level higher than upper limit of normal range.
Serum calcium level above upper limit of normal (> 10 mg/dL) corrected for serum albumin, or ionized calcium level higher than upper limit of normal range.
![]() Increased 24-hour urine calcium excretion (> 200 mg).
Increased 24-hour urine calcium excretion (> 200 mg).
![]() Elevated alkaline phosphatase.
Elevated alkaline phosphatase.
![]() Decreased serum phosphate level.
Decreased serum phosphate level.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


