Endocrine Systems and the Heart
Marco Roffi
Fabio Cattaneo
The Thyroid
The major secretory product of the thyroid gland is thyroxine (T4), a relatively inactive hormone. Subsequently, T4 is converted by the enzyme 5′-monodeiodinase to the biologically active compound triiodothyronine (T3). Both thyroid hormone excess and depletion may affect the cardiovascular system. Cardiovascular involvement in thyroid dysfunction may be the result of direct hormone effects at the cellular level, interactions with the sympathetic nervous system, or alterations of peripheral circulation and metabolism (1). At the cellular level, thyroid hormones act mainly through binding to specific nuclear receptors and activation of gene transcription. Additionally, they activate extranuclear sites as mitochondrial- and membrane-bound enzymes (1). Women are more likely to develop thyroid dysfunction, with an estimated 2.7% prevalence of hyperthyroidism and 1.9% prevalence of hypothyroidism in an unselected population (2).
Thyrotoxicosis
The terms hyperthyroidism and thyrotoxicosis can be differentiated, but are frequently used interchangeably. Hyperthyroidism is increased formation and release of thyroid hormones from the thyroid gland, whereas thyrotoxicosis is the clinical syndrome that results from thyroid hormone excess. The most frequent cause of thyrotoxicosis is Graves disease, which accounts for 60% to 90% of cases and occurs in women 10 times more frequently than in men. This disorder is characterized by autoantibodies activating the thyroid-stimulating hormone (TSH) receptor. Other causes of thyrotoxicosis include toxic adenoma, toxic multinodular goiter, thyroiditis, and thyroid autonomy.
Cardiovascular symptoms of thyroid hormone excess are nonspecific. Palpitations are usually caused by sinus tachycardia and, occasionally, by atrial fibrillation. Exercise intolerance and dyspnea on exertion may be a result of the combination of inability to raise cardiac output and skeletal and/or respiratory muscle weakness. The hemodynamic changes occurring in thyrotoxicosis are summarized in Table 35A.1 and include tachycardia, increased cardiac output and stroke volume, and decreased systemic vascular resistance (3,4). In the elderly, cardiovascular involvement may be limited to arrhythmias such as sinus tachycardia or atrial fibrillation, which occasionally trigger angina or heart failure. In contrast to hypothyroidism, which is characterized by diastolic hypertension, hyperthyroidism is associated with systolic hypertension in the presence of normal or low diastolic blood pressure. It is speculated that isolated systolic hypertension is secondary to the inability of the vasculature to accommodate increased cardiac output and stroke volume. Several studies have demonstrated that hyperthyroidism is associated with increased cardiovascular morbidity and mortality. In an English cohort of 7,203 patients treated for hyperthyroidism the standardized mortality ratio was 1.1 in comparison with the general population and an excess of cardiovascular deaths was identified (5). Similarly, a standardized mortality rate of 1.3 over 14 years was identified among 1,762 women treated for hyperthyroidism at a U.S. center (6). Moreover, a Swedish study following 10,522 patients for 15 years after radioiodine treatment detected a standardized mortality rate of 1.5 for women and 1.3 for men (7). Even in the absence of symptoms, low TSH has been identified as an independent predictor of mortality (2.1 standardized mortality ratio) among 1,191 individuals followed for 10 years (8). The observed increase was largely accounted for by cardiovascular mortality.
Sympathetic Nervous System and Ventricular Function
Many of the cardiovascular signs and symptoms of thyrotoxicosis mimic a high-adrenergic state and respond to β-blockade, suggesting an underlying dysfunction of the catecholamine metabolism or, alternatively, an increased sensitivity to catecholamines. However, patients with thyrotoxicosis have low or normal plasma catecholamine levels, normal urinary catecholamine excretion, and normal response to catecholamine infusion (9). In addition, there is no conclusive evidence of increased β-adrenergic receptor density in the myocardium, increased catecholamine turnover at neural synapses, or increased affinity of adrenergic receptor for catecholamines. Finally, recent animal studies support the notion that the cardiovascular effects of hyperthyroidism are largely independent from adrenergic activation (10).
Short-term hyperthyroidism is associated with increased cardiac contractility and improved diastolic function, which
may be the result of augmented activity of the sarcoplasmic reticulum calcium ATPase pump (11). In both humans and animals, chronic thyrotoxicosis causes variable degrees of left ventricular hypertrophy (LVH). It has been demonstrated that thyroid hormones induce cardiac protein synthesis, leading to the hypothesis that this anabolic pathway may be the trigger of LVH (12). However, β-adrenergics effectively block or reverse hypertrophy, suggesting that increased cardiac workload is the mediator of LVH (13). In addition to the effects on the myocardium, T3 shows vasodilator properties by acting directly on vascular smooth muscle cells, potentially explaining the decreased systemic vascular resistance observed in hyperthyroidism (14).
may be the result of augmented activity of the sarcoplasmic reticulum calcium ATPase pump (11). In both humans and animals, chronic thyrotoxicosis causes variable degrees of left ventricular hypertrophy (LVH). It has been demonstrated that thyroid hormones induce cardiac protein synthesis, leading to the hypothesis that this anabolic pathway may be the trigger of LVH (12). However, β-adrenergics effectively block or reverse hypertrophy, suggesting that increased cardiac workload is the mediator of LVH (13). In addition to the effects on the myocardium, T3 shows vasodilator properties by acting directly on vascular smooth muscle cells, potentially explaining the decreased systemic vascular resistance observed in hyperthyroidism (14).
TABLE 35A.1 Cardiovascular Hemodynamics in Thyroid Dysfunction | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
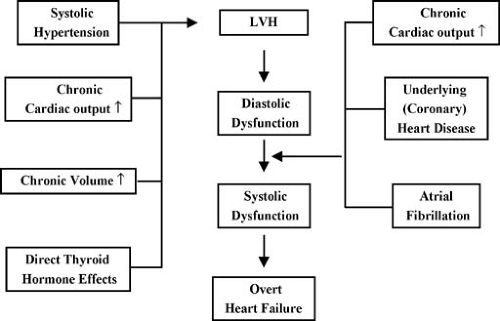
It remains a source of debate whether thyrotoxicosis per se may lead to heart failure. In the majority of cases, heart failure can be explained by the combination of underlying heart disease, arrhythmias, and chronically increased cardiac output. However, myocardial dysfunction has been described in absence of underlying cardiac disease (15) and improvement in myocardial contractility after restoration of euthyroidism has been reported (16). Therefore, thyroid hormone excess should be excluded in patients with unexplained heart failure. Several factors may contribute to heart failure in thyrotoxicosis (3,4). Diastolic function deteriorates in the course of the disease owing to LVH and progressive left ventricular (LV) stiffness, leading to LV filling impairment, in particular in the setting of tachycardia or atrial fibrillation (Fig. 35A.1). In addition, thyrotoxic patients may present with intravascular volume expansion, possibly secondary to a decrease in renal perfusion with subsequent renin-angiotensin system-mediated increase in sodium reabsorption (17). Occasionally, the decreased systemic resistance may overwhelm the cardiac capacity and cause high output failure. More frequently however, the high-output state may unmask coronary artery disease, and heart failure is precipitated by ischemia.
Arrhythmias
Sinus tachycardia at rest, during sleep, and during exercise is the most common arrhythmia in thyrotoxicosis. Other common rhythm disturbances include atrial premature contractions and atrial fibrillation. Less frequently, patients present with paroxysmal atrial tachycardia and atrial flutter. Ventricular arrhythmias are rare. It is speculated that thyroid hormones have direct effects on the conduction system, possibly via cellular changes in cation transport, this may trigger a decrease of atrial excitation threshold, an increase of sinoatrial node firing, and the shortening of conduction tissue refractory with subsequent rapid ventricular rate response in the presence of supraventricular arrhythmias (18). Although thyrotoxicosis is the underlying etiology in less than 5% of atrial fibrillation cases, this rhythm disturbance may occur in 5% to 15% of hyperthyroid patients. In one recent population-based study that included 40,628 patients with clinical hyperthyroidism, atrial fibrillation or flutter were found in 8.3% of cases (19). Atrial fibrillation related to thyrotoxicosis is more frequently detected in the elderly and in males, probably reflecting the increased prevalence of intrinsic heart disease. Because atrial fibrillation, particularly in the elderly, may be the only manifestation of thyrotoxicosis, thyroid hormone excess should be excluded in this setting. Accordingly, one report showed subtle hyperthyroidism in 12.5% of elderly patients with atrial fibrillation previously considered idiopathic (20). Major complications of atrial fibrillation include heart failure and embolic events. In the absence of chronic atrial fibrillation or underlying heart disease, most patients convert spontaneously to sinus rhythm within 8 to 12 weeks of antithyroid treatment (21). Conversely, reversion into atrial fibrillation is likely in persistently thyrotoxic patients. Patients in atrial fibrillation should be anticoagulated and cardioversion should be deferred until euthyroidism is restored.
Subclinical Hyperthyroidism
Cardiovascular manifestations of subclinical hyperthyroidism include sinus tachycardia, LVH, diastolic dysfunction, reduced
exercise performance, and atrial fibrillation (22). A study including 2,002 patients older than 60 years of age with serum TSH levels of 0.1 mU/L or lower reported a threefold increased risk of atrial fibrillation over 10 years (23). Similarly, in a cross-sectional study involving 23,638 patients, low serum TSH was associated with a fivefold higher prevalence of atrial fibrillation compared with individuals with normal levels (13.3% and 2.3%, respectively), with no difference between subclinical or overt thyroid hormone excess (24). Recently proposed guidelines for the diagnosis and management of subclinical thyroid disease have summarized the impact of subclinical hyperthyroidism on cardiovascular outcomes and the benefit of treatment (22). The investigators, underscoring the paucity of data, advised against routine treatment for patients whose TSH is mildly decreased (serum TSH 0.1–0.45 mU/L), even in the presence of atrial fibrillation. Accordingly, there is only limited evidence that antithyroid therapy may facilitate spontaneous conversion to sinus rhythm or be helpful in maintaining sinus rhythm following cardioversion in this setting.
exercise performance, and atrial fibrillation (22). A study including 2,002 patients older than 60 years of age with serum TSH levels of 0.1 mU/L or lower reported a threefold increased risk of atrial fibrillation over 10 years (23). Similarly, in a cross-sectional study involving 23,638 patients, low serum TSH was associated with a fivefold higher prevalence of atrial fibrillation compared with individuals with normal levels (13.3% and 2.3%, respectively), with no difference between subclinical or overt thyroid hormone excess (24). Recently proposed guidelines for the diagnosis and management of subclinical thyroid disease have summarized the impact of subclinical hyperthyroidism on cardiovascular outcomes and the benefit of treatment (22). The investigators, underscoring the paucity of data, advised against routine treatment for patients whose TSH is mildly decreased (serum TSH 0.1–0.45 mU/L), even in the presence of atrial fibrillation. Accordingly, there is only limited evidence that antithyroid therapy may facilitate spontaneous conversion to sinus rhythm or be helpful in maintaining sinus rhythm following cardioversion in this setting.
Diagnosis and Therapy
Measurement of serum TSH is the most sensitive screening test for hyperthyroidism. An undetectable value is the hallmark of the disease, whereas normal TSH virtually excludes it. Elevated serum levels of free T4, free T3, or total T3 confirm the diagnosis. β-Adrenergic blockers provide relief of symptoms such as tachycardia, tremor, anxiety, and heat intolerance. Alternatively, calcium channel blockers such as verapamil or diltiazem have been administered. However, caution is warranted, because these agents may cause hemodynamic instability by further reducing systemic vascular resistance and contractility. The discussion of therapy strategies to reduce thyroid hormone synthesis is beyond the scope of the chapter. It remains to be determined whether an early and aggressive control of hyperthyroidism may positively influence the increased morbidity and mortality associated with this condition. Encouraging are the findings of a large-scale study documenting that the cardiovascular mortality excess noted among hyperthyroid patients undergoing radioiodine treatment was highest in the first year following treatment and then declined (5).
Hypothyroidism
Hypothyroidism is the clinical syndrome associated with decreased secretion of thyroid hormones. This condition reflects in over 90% of cases a disease of the gland itself (primary hypothyroidism). Rarely, hypothyroidism can be caused by pituitary disease (secondary hypothyroidism) or hypothalamic disease (tertiary hypothyroidism). The most frequent cause of hypothyroidism in adults is autoimmune thyroiditis, or Hashimoto disease, which affects mainly older women. The slow and progressive nature of hypothyroidism makes the diagnosis difficult, particularly in the elderly. Most hypothyroid patients present with nonspecific symptoms caused by psychological or skeletal muscle dysfunction.
Longstanding hypothyroidism may affect the cardiovascular system. Bradycardia is common. Pericardial effusion may occur, but rarely causes hemodynamic compromise. Both systolic and diastolic LV performance may be decreased, presumably because of alterations in calcium uptake and release by cardiac myocytes (4). An increase in systemic vascular resistance has been described, possibly as the result of the lacking direct vasodilatory effect of thyroid hormones (14). Despite symptoms suggesting a decreased sympathetic tone, plasma catecholamines are increased (25). The resulting hemodynamic changes are opposite but less marked than in thyrotoxicosis (see Table 35A.1). Characteristic features include diminished cardiac output, decreased stroke volume, impaired ventricular function, decreased intravascular volume, increased systemic vascular resistance, and decreased peripheral oxygen consumption (26). Heart failure may occur when the metabolic demand cannot be matched by adequate cardiac output. As in patients with thyrotoxicosis, overt heart failure in hypothyroidism generally represents exacerbation of intrinsic cardiac disease. Rarely, thyroid hormone depletion may cause cardiomyopathy. Therefore, unexplained heart failure should prompt determination of thyroid hormones. In the absence of underlying heart disease, the decreased myocardial contractility observed in hypothyroidism may be reversible after hormone replacement.
Coronary Artery Disease
Patients with hypothyroidism are burdened by an increased prevalence of hyperlipidemia, hypertension, and atherosclerosis. Total cholesterol, low-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol, lipoprotein (a), and apolipoprotein B concentrations are often elevated in hypothyroidism, and some patients have high serum triglycerides. It has been demonstrated that patients with hypothyroidism have an intrinsic low-density lipoprotein cholesterol catabolism dysfunction, which is reversible after hormone replacement (27). In a review of 12 studies, the prevalence of hypertension in this patient population was 21% (28). In large series of hypertensive patients, hypothyroidism accounted for 3% to 5% of the cases (29,30). Although the pathophysiology remains unknown, a causal link between thyroid hormone deficiency and hypertension is confirmed by the observation that hormone replacement may lower blood pressure in these patients (30




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree