Chapter 27 Electrophysiological Evaluation of Atrial Tachycardia and Atrial Flutter
Atrial Tachycardia
Focal atrial tachycardia (AT) is the least common type of supraventricular tachycardia (SVT), comprising approximately 10% to 15% of patients referred for electrophysiological evaluation of SVT.1 Possible mechanisms include triggered activity, enhanced automaticity, or micro–re-entry. AT has been defined as rhythmic atrial activation spreading centrifugally from a focal area less than 2 cm.2 Although no difference in prevalence exists between genders and any age group may be affected, some evidence suggests that older patients are more likely to have multiple foci from the right atrium that have a nonautomatic mechanism and are recurrent after catheter ablation.3
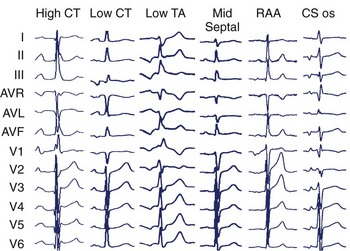
It is well recognized that focal ATs are not randomly distributed but, rather, congregate around defined anatomic locations. In the right atrium, the most common site is along the crista terminalis (CT), with foci also clustering around the tricuspid annulus, coronary sinus os, right atrial appendage, perinodal region, and inter-atrial septum.4–7 In the left atrium, the pulmonary veins provide the majority of foci, with the mitral annulus, left septum, left atrial appendage, and noncoronary aortic cusp being other potential locations.8–12 Knowledge of this clustering is of great assistance to the electrophysiologist while attempting to localize the likely site of origin during an electrophysiology study.
Indications for Referral to the Electrophysiology Laboratory
Although focal atrial ectopy is common, it is rarely symptomatic. In contrast, frequent ectopy and sustained tachycardia are often symptomatic and prompt patients to present to the clinician for assessment. Incessant AT may also induce left ventricular dysfunction, which can resolve after curative ablation3 Focal AT tends to respond poorly to pharmacologic therapy, making catheter ablation an increasingly used procedure. The American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the European Society of Cardiology Committee (ACC/AHA/ESC) consensus guidelines published in 2003 provide a class I recommendation for catheter ablation of focal AT in patients with incessant tachycardia or recurrent symptoms.1
Importance of Surface Electrocardiogram and P-Wave Morphology
The surface electrocardiogram (ECG) can be a useful guide to the likely anatomic site of origin for focal AT. This information can then be used by the clinician during intracardiac electrophysiological mapping to target specific areas. Kistler et al published an algorithm for the localization of atrial tachycardias based on the surface ECG P-wave morphology (PWM) that was able to identify the correct anatomic location with an accuracy of 93%.13 PWM is typically described as positive, negative, isoelectric, bifid, or biphasic (positive-negative or negative-positive). When analyzing PWM, it is important to ensure that the preceding T wave is not superimposed. Spontaneous ventricular ectopic beats, programmed ventricular extrastimuli, or vagal maneuvers such as the Valsalva maneuver or carotid sinus massage may assist with separation of the T wave and P wave. Distinguishing left and right atrial foci is of importance when planning a procedure, as trans-septal access may be anticipated on the basis of PWM. A left-sided focus is suggested by a positive P wave in V1 and a negative P wave in I and aVL.
Specific anatomic sites have characteristic PWMs. Examples of right atrial foci PWMs are shown in Figure 27-1. Foci from the crista terminalis account for two thirds of right atrial foci.4 The characteristic PWM is similar to the sinus rhythm being positive-negative in V1, positive in I and II, and negative in aVR.13 Foci from the tricuspid annulus (TA) tend to have a negative P wave in V1 and V2, with superior locations displaying positive PWM in II, III, and aVF and inferior locations displaying negative PWM in the inferior leads. Right atrial appendage locations have indistinguishable PWM to superior TA locations. Focal ATs from the coronary sinus (CS) ostium are characterized by deeply negative PWM in the inferior leads, positive in aVL and aVR, and isoelectric-positive or negative-positive in V1. Septal locations may have variable PWM.
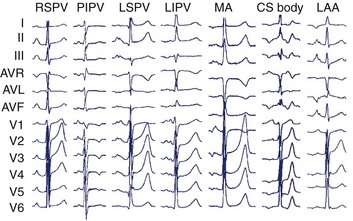
In the left atrium, pulmonary veins are the most common sources of foci. Representative left atrial PWMs are shown in Figure 27-2. V1 is typically positive across the chest leads, aVR is negative, and aVL is isoelectric or negative.13 Right and left superior pulmonary veins (PVs) tend to have positive PWM in the inferior leads; conversely, right and left inferior PVs have low-amplitude or inverted P waves inferiorly. Left-sided veins exhibit a broader notched P wave in V1 and in the inferior leads, compared with right-sided veins. The left atrial appendage (LAA) foci have a PWM similar to that of the left superior PV, although a deeply negative P wave in lead I, particularly in the setting of incessant tachycardia, is a strong clue that this may be an LAA focus.11 Mitral annular locations near the aorto-mitral continuity tend to have biphasic negative-positive PWM in V1 and isoelectric or negative PWM in aVL. More recently, focal AT from the noncoronary cusp of the aortic valve has been described, with a PWM similar to the mitral annular location.9 A positive P wave in leads I and aVL may help differentiate this site from the mitral annular location.
Confirming the Diagnosis
The diagnosis of focal AT can usually be made on the surface ECG. Important differential diagnoses include sinus tachycardia (ST), atrioventricular node re-entry tachycardia (AVNRT), atrioventricular reciprocating tachycardia (AVRT), and macro–re-entrant AT. On the surface ECG, AT typically manifests with a long R-P interval. However, at rapid rates, decremental AV nodal conduction may result in an apparent short R-P tachycardia. While both typical AVNRT and AVRT characteristically present with a short R-P interval, this relationship is fixed or “hooked.” In focal AT, a variable or unhooked R-P relationship may be present. When clearly not of sinus origin, PWM can be used to distinguish AT from ST. However, AT arising from the superior crista terminalis may have a morphology indistinguishable from that of ST. Indeed, these tachycardias were previously termed “sinus node re-entry.” The presence of a “warm-up” over three to four beats at onset and “cool-down” over three to four beats at termination supports a diagnosis of AT over ST, which typically accelerates and decelerates over more than 30 seconds.14 Macro–re-entrant AT generally manifests with an undulating baseline and no clear isoelectric segment (which is usually seen in focal AT). It is important to note that focal tachycardias at very short cycle lengths may show no isoelectric baseline and mimic macro–re-entry. Similarly, focal tachycardia in atria with scarring and slowed conduction may also show an undulating baseline because of circuitous propagation away from the focus.
Confirming the Diagnosis Using Intracardiac Recordings and Pacing Maneuvers
Although the surface ECG may have provided a likely diagnosis, intracardiac recordings and pacing maneuvers during tachycardia are often required to exclude alternative diagnoses. On examination of the intracardiac recordings, the relationship between the ventricular and atrial electrograms (VA relationship) is seen to be fixed or “hooked” in AVNRT and AVRT but variable in AT. Various pacing maneuvers may facilitate the observation of VA unhooking. Following termination of the atrial overdrive pacing at a cycle length that is just below the tachycardia cycle length, the VA relationship remains fixed in both AVNRT and AVRT; however, VA unhooking is observed when the mechanism is focal AT.15 The response after entrainment of the tachycardia by ventricular pacing (confirmed by acceleration of the atrial rate to the pacing rate) is also helpful. A “V-A-V” response is characteristic of AVRT or AVNRT, and a “V-A-A-V” response is observed in AT. In addition, AT may be excluded if termination of the tachycardia occurred without atrial depolarization during burst ventricular pacing at a 200- to 250-ms cycle length for three to six beats. Demonstration of entrainment from “within the circuit” (Post-pacing interval—Tachycardia cycle length [PPI — TCL] <20 ms) from two sites more than 2 cm apart or the ability to record activity throughout the entire tachycardia cycle length are both definitive for macro–re-entrant AT.1
Conventional Mapping
The hallmark of focal AT is the identification of a focal site of early activation with centrifugal spread on endocardial mapping. A local activation time of more than 20 to 30 ms before the surface ECG P-wave onset indicates a potential site for successful ablation.16 Attempts to define the clear P-wave onset (using pacing maneuvers or adenosine if it cannot be seen) are important. The P-wave onset can then be referenced to a stable intracardiac reference point such as the proximal CS bipole.
Multipolar catheters (20-pole TA or CT) may act as a guide to the focal origin and may also be helpful to rapidly recognize any change in the mechanism or the site of origin of the tachycardia. Pace mapping may also be used to complement activation sequence mapping. The endocardial activation sequence during pacing from various sites that has been mapped by using a catheter can be compared with the spontaneous activation sequence seen during tachycardia.17
Electrogram Characteristics of Successful Sites
Several groups have reported on the presence of electrogram fractionation recorded from ablation catheters at the sites of successful ablation. However, this finding is not universal and may be specific to certain anatomic regions only (e.g., electrograms for focal AT arising from the CT commonly show fractionation, but those at the tricuspid and mitral annulus usually do not).16 It is hypothesized that these fractionated signals represent underlying slow conduction caused by impaired intercellular coupling, which results in the potential for micro–re-entry.
Many electrophysiological laboratories use the unipolar electrogram at the ablation catheter tip to identify the characteristic QS pattern of a successful ablation site.18
The termination of sustained tachycardia with pressure from an intracardiac catheter has been reported to improve on the specificity and positive predictive value of identifying an AT focus.19 In practice, if ablation is performed at a site of termination by catheter pressure, it may be uncertain whether ablation has been successful or if mechanical pressure has simply caused transient stunning of the focus. In the experience of the authors, ablation at the site of mechanical termination carries a relatively high risk of recurrence.
Atrial pacing during tachycardia at a rate just below the tachycardia cycle length has been used to identify the site of focal origin.20 In theory, at the site of origin the PPI-TCL should equal 0 ms. In a recent study, the postpacing interval minus the tachycardia cycle length was 11 ± 8 ms at sites of successful ablation.
Three-Dimensional Electroanatomic Mapping Systems
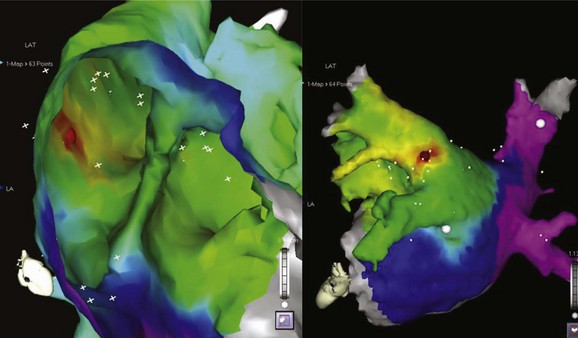
The development of three-dimensional mapping systems has significantly improved the ability of clinicians to successfully locate and ablate foci. Both the CARTO (Biosense Webster, Johnson & Johnson, Diamond Bar, CA) and NavX (Diag Corporation, Minnetonka, MN) systems use complex algorithms for the registration of a mapping/ablation catheter position in three-dimensional space relative to a fiducial point. This allows the construction of three-dimensional chamber geometry with activation sequence mapping (traditionally color coded). In addition, these data can be superimposed onto images of the atrium taken by either computed tomography or magnetic resonance imaging (MRI) scans allowing detailed and accurate correlation of the atrial anatomy with intracardiac electrograms (for an example, see Figure 27-3). Several groups have verified the usefulness of these systems in locating and ablating AT foci.16 Despite these advantages and the potential for a reduction in radiation exposure because of lower fluoroscopy times, these mapping systems still require frequent ectopy or sustained tachycardia for effective mapping.
Ablation
Standard radiofrequency ablation catheters may be used in the vast majority of cases; irrigated tip catheters may be useful in areas of the atrium with slow blood flow, such as trabeculated areas and within the CS. Cryoablation is an alternative energy source with the advantage of reversibility at –30° C. Moreover, once applied at this temperature, the catheter remains firmly adherent to the underlying tissue, providing excellent catheter stability. These attributes make this energy source an ideal choice for perinodal foci, where permanent AV-nodal block can be avoided by assessing for block with a reversible lesion before applying a full lesion at –80° C (−112° F).21 Long vascular sheaths may assist with catheter direction, contact, and stability.
Overall, the success rates for catheter ablation are excellent; reported series cite cure rates between 69% and 100%.16 On the basis of pooled data, recurrence was predicted to be approximately 7%.3 Factors predicting recurrence in this analysis included male gender, multiple foci, older age, and coexisting cardiac pathology. Right atrial foci were predicted to have higher success rates.
Macro–Re-entrant Atrial Tachycardia
Definitions
Macro–re-entrant AT is a broad term that encompasses both typical (cavo-tricuspid isthmus [CTI])–dependent) atrial flutter and atypical (non–CTI-dependent) atrial flutter (or macro–re-entrant tachycardias). They have in common the classic requirements for re-entry: obstacles which create areas of anatomic or functional conduction block around which two pathways may conduct; unidirectional block in one pathway; and areas of slow conduction creating an excitable gap. Classic entrainment identifies areas “within” the circuit and should be demonstrable by pacing from at least two sites more than 2 cm apart and finding a postpacing interval minus tachycardia cycle length of 20 ms or less. Identification of a critical isthmus by demonstration of concealment (whether on the surface or by intracardiac recordings) is fraught with problems and is of limited practical utility in the atrium.2
Typical Atrial Flutter
Typical atrial flutter is the most common form of macro–re-entrant AT and typically has a cycle length of approximately 200 ms. This may be considerably longer (ranging up to 300 ms) in the presence of atrial disease with atrial enlargement and conduction slowing, particularly in the presence of antiarrhythmic agents which slow conduction. Its prevalence is estimated at 5 per 100,000 in those younger than 50 years and increases with age to almost 600 per 100,000 among those older than 80 years.22 Men are affected 2.5 times more frequently than are women.22 The anatomic boundaries of this circuit are well known and are described elsewhere in this text (see Chapter 44).
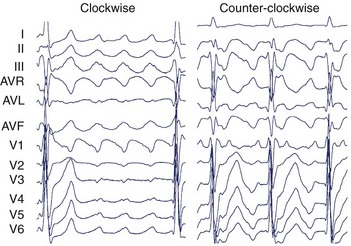
The surface ECG of typical CCW flutter is characterized by isoelectric-positive P waves in V1 transitioning to negative across the precordium (Figure 27-4). Flutter waves in the inferior leads are generally deeply inverted and have a variable secondary upright component timing with activation of the atrial free wall from superior to inferior. When this secondary component is prominent, it may be difficult to determine whether the flutter wave is predominantly “up” or “down.” The ECG appearance of clockwise typical CTI-dependent flutter is more variable. Commonly, V1 is broad and negative with notching, transitioning to positive across the precordium; the inferior leads are usually broad and positive with notching (see Figure 27-4).23 Other macro–re-entrant circuits have variable ECG characteristics and, as such, detailed intracardiac electrophysiological studies are required for accurate anatomic localization.
Indications for Catheter Ablation
In general, pharmacologic therapy for atrial flutter is either targeted at ventricular rate control using AV-nodal blocking agents (such as β-blockers and cardiac-selective calcium channel blockers) or maintenance of sinus rhythm with class I and III antiarrhythmic drugs. Both these approaches have limitations; the overall maintenance of sinus rhythm on antiarrhythmic drugs is between 36% and 73%.1 Evidence from a randomized prospective study of catheter ablation versus medical therapy demonstrated sinus rhythm at 21 ± 11 months in 80% of patients with catheter ablation, compared with 36% for those on antiarrhythmic drugs.24 In addition, the ablation group had significantly fewer hospital admissions and episodes of atrial fibrillation (AF). For patients with coexisting AF, in whom flutter is the dominant rhythm, catheter ablation has been shown to reduce the burden of flutter and assist pharmacologic control of AF.1 The ACC/AHA/ESC consensus guidelines provide a class I recommendation for catheter ablation of atrial flutter if it is recurrent, is poorly tolerated, or re-emerges after class I antiarrhythmic or amiodarone therapy.1 A class IIa recommendation is given for atypical flutter after failure of antiarrhythmic medications.
Anatomy of the Cavo-Tricuspid Isthmus
The anatomy of the CTI has been described as a quadrilateral structure bounded anteriorly by the TA and posteriorly by the eustachian ridge. It is limited laterally by the terminal insertion of the crista terminalis and medially by the CS ostium and the thebesian valve. Trabeculae within the CTI are formed by pectinate extensions of the crista terminalis and intervening connective tissue.25–27 The thicknesses of CTI trabeculae and the eustachian ridge vary markedly, being largely fibrous tissue with sparse muscle fibers in some patients and deeply trabeculated with thick muscle bundles and intervening pouches or recesses in others.28 It is this variability in anatomy that determines the ease or difficulty of ablation. The CTI tends to be wider more laterally than in the middle or septal regions. The septal aspect of the CTI lies close to the posterior extensions of the AV node and to the middle cardiac vein.28 The smooth vestibular component around the tricuspid valve orifice lies immediately over the right coronary artery.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree