Chapter 51 Electrocardiographic Manifestations of the Athlete’s Heart and Management of Arrhythmias in the Athlete
Introduction
Participation in regular physical exercise confers well-established benefits with respect to cardiovascular health.1–3 Paradoxically, young athletes represent a group of individuals who may be particularly vulnerable to the risk of sudden cardiac death (SCD) from unrecognized inherited or acquired disorders of the heart (Table 53-1).4 SCD in young trained individuals is rare, with an estimated incidence ranging from 1 in 50,000 to 1 in 200,000 patient-years. However, SCDs often receive considerable media attention and have a profound impact on the local, lay, and sporting communities, which perceive young asymptomatic athletes to represent the healthiest segment of society.5 The youth of the individual, the potential life years lost, and the ability to diagnose most disorders capable of causing sudden death often galvanize emotionally charged and controversial debates among the lay community, medical bodies, and sporting organizations relating to the practical use of pre-participation screening to identify disorders capable of causing SCD. The role of the 12-lead electrocardiogram (ECG) as a screening tool for the diagnosis or identification of athletes requiring further investigation is established in many European countries and is practiced among elite and financially endowed sporting organizations in the United States, although the U.S. guidelines do not currently advocate a mandatory ECG as part of pre-participation screening in athletes in general. Indeed, electrocardiographic screening of athletes is endorsed by the European Society of Sport Cardiology, the International Olympic Committee, and the Fédération Internationale de Football Association (FIFA).6,7 However, athletes often exhibit anomalous ECG patterns relating to increased vagal tone and chamber size as a consequence of physiological adaptations that overlap with certain disease phenotypes implicated in SCD.8–11 The differentiation between physiology and pathology is crucial, and an erroneous diagnosis has the potential for serious consequences. This chapter provides a comprehensive overview of the resting ECG in athletes to facilitate the differentiation between physiological changes and pathologic disorders and identifies the prevalence, significance, and management of cardiac arrhythmias in athletes, based on guidelines developed by expert consensus panels in Europe (ESC consensus statement) and the United States (the 36th Bethesda Conference guidelines).12–14
Table 51-1 Causes of Sudden Cardiac Death in Athletes Younger Than 35 Years in the United States Between 1986 and 2007
| DIAGNOSIS AT POSTMORTEM | FREQUENCY (%) |
|---|---|
| Hypertrophic cardiomyopathy | 36 |
| Coronary artery anomalies | 17 |
| Myocarditis | 5.9 |
| Arrhythmogenic right ventricular cardiomyopathy | 4.3 |
| Ion channelopathies (long QT and Brugada syndromes) | 3.6 |
| Mitral valve prolapse | 3.4 |
| Myocardial bridging | 3.3 |
| Premature coronary artery disease | 3.3 |
| Aortic stenosis | 2.4 |
| Marfan’s syndrome | 2.7 |
| Idiopathic dilated cardiomyopathy | 2.0 |
| Wolff-Parkinson-White syndrome | 1.6 |
From Maron BJ: Sudden death in young athletes, N Engl J Med 349:1064–1075, 2003.
The Athlete’s Heart
Common Findings on the Resting 12-Lead Electrocardiogram
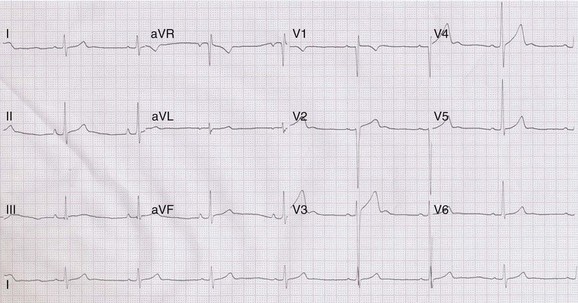
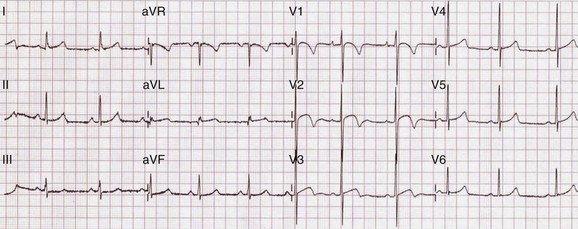
Regular participation in intensive physical exercise is associated with central and peripheral cardiovascular adaptations to facilitate the generation of a large and sustained cardiac output and to enhance the extraction of oxygen from exercising muscle for aerobic glycolysis, respectively. Collectively, these physiological adaptations have been termed the athlete’s heart.15 Specific structural and electrical cardiac remodeling is fundamental to the physiological adaptive process, and most athletes exhibit modest increases in chamber wall thickness and cavity size, as well as changes in parasympathetic and sympathetic tone, as reflected on the surface 12-lead ECG (Table 51-2 and Figure 51-1).16,17
Table 51-2 Common Findings in the Resting 12-Lead ECG in the Athlete
| ECG FINDING | FREQUENCY (%) |
|---|---|
| Sinus bradycardia | 80 |
| Sinus arrhythmia | 52 |
| First-degree AV block | 5 |
| Incomplete RBBB | 29 |
| LA enlargement | 14 |
| RA enlargement | 16 |
| Sokolow-Lyon criteria for LVH | 45 |
| ST elevation | 43 |
| Large-amplitude T waves | 22 |
ECG, Electrocardiogram; AV, atrioventricular; RBBB, right bundle branch block; LA, left atrial; RA, right atrial; LVH, left ventricular hypertrophy.
From Sharma S, Whyte G, Elliott P, et al: Electrocardiographic changes in 1000 highly trained junior elite athletes, Br J Sports Med 33:319–324, 1999.
Cardiac Rhythm
Common rhythmic ECG findings in athletes include sinus bradycardia, which is present in up to 80% of highly trained athletes, sinus arrhythmia (52%), and first-degree heart block (5%).18 Occasionally, higher levels of atrioventricular (AV) nodal block may be observed.19 Such rhythm anomalies are considered secondary to increased vagal tone. In addition, desensitization of β-receptors and changes in the intrinsic properties of the sinoatrial (SA) and AV nodes in response to physical training may also contribute.20,21 The maximum heart rate is unaffected, and heart block during resting conditions generally resolves immediately on initiation of exercise.18
Electrical Voltage Criteria for Cardiac Enlargement
High-magnitude QRS voltages, particularly across the precordial leads, are common manifestations of athlete’s heart and may be identified in up to 60% of athletes.8 Increased QRS voltage criteria can only be partly attributed to a direct consequence of increased cardiac size. In general, poor correlation exists between voltage criteria for cardiac hypertrophy and cardiac dimensions identified at echocardiography. Most correlation studies relate specifically to the assessment of left ventricular hypertrophy either in terms of absolute left ventricular wall thickness or left ventricular mass measurements at echocardiography; these studies demonstrate poor sensitivity and specificity for the identification of left ventricular hypertrophy at echocardiography for the vast majority of voltage criteria used.8,11 In our experience, the Sokolow-Lyon voltage criterion for left ventricular hypertrophy, which is the most commonly used in clinical practice, has a sensitivity and specificity of 16% and 60%, respectively, for the identification of left ventricular hypertrophy. Several other factors influence QRS voltage magnitude, including the distance between the heart and the thoracic wall, which is determined by chest wall size, pectoral hypertrophy, and the presence or absence of breast tissue. In contrast, the more stringent electrocardiographic Romihilt-Estes points score of 5 or greater for left ventricular hypertrophy is a less frequent observation in athletes (10% of male athletes) but has a specificity for left ventricular hypertrophy in the range of 90%.8
The presence of voltage criteria for right ventricular hypertrophy is less common in athletes; however, incomplete right bundle branch block (RBBB) has been documented in up to 29% of highly trained athletes and is thought to represent mild increases in right ventricular size.8 Similarly, voltage criteria for left and right atrial enlargement are common findings, present in 14% and 16% of adolescent athletes, respectively.8 As with voltage criteria for ventricular hypertrophy, the sensitivity and specificity of such voltage scores to predict the presence of left atrial enlargement is poor, with only 0.3% of 347 adult athletes with echocardiographic evidence of left atrial enlargement having positive voltage criteria.22
Repolarization Changes
Early repolarization changes are common in athletes and are observed in 40% to 50% of individuals.8 Specific anomalies include J-point and ST-segment elevations and increased T-wave voltages (>0.2 mV), which reflect modulations in autonomic tone affecting the timing sequence between depolarization and repolarization. The anomalies resolve at increased heart rates during gentle exercise, regress with detraining, and are considered benign.
Impact of Demographic Factors and Sporting Discipline on the Athlete’s Electrocardiogram
Age
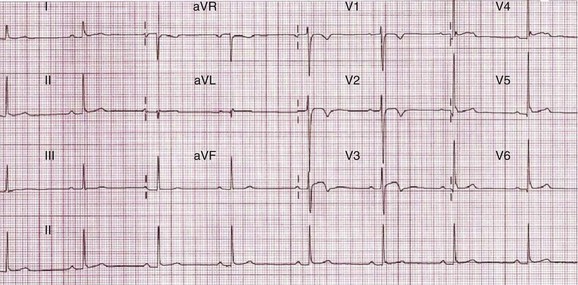
In general, the qualitative changes that occur in adolescent athletes are similar to those observed in more mature adults. However, voltage criteria for left and right ventricular hypertrophy may be more commonly present in adolescent athletes because of their thin chest walls.8 T-wave inversions in the right ventricular leads (V1 to V3) may be observed in up to 5% of adolescent athletes and usually regress by the age of 16 years and are representative of the juvenile ECG pattern rather than cardiac pathology (Figure 51-2).23 In contrast, T-wave inversions in the anterior leads beyond V1 are identified in only 0.1% of adult athletes and warrant further investigations to exclude a cardiac disorder.23
Ethnicity
The concept that an athlete’s ethnic background may alter the appearance of the resting 12-lead ECG has been examined by a number of recent reports comparing ECG patterns between white athletes and those with African or Afro-Caribbean ancestry (referred to as black athletes). Racial differences in ECG appearance have been recognized historically, with the observation in the 1950s that J-point and ST-segment elevation may be part of the normal ECG in young black men.24,25
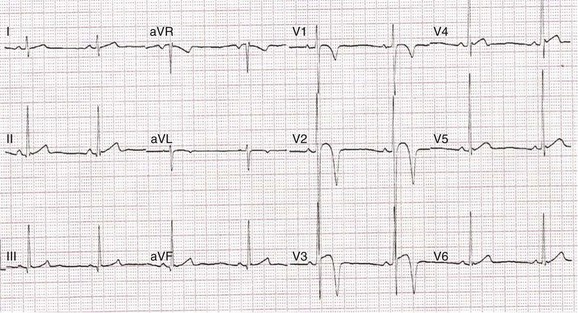
Recent studies have demonstrated that black male athletes demonstrate a higher prevalence of voltage criteria for left ventricular hypertrophy as well as bizarre repolarization anomalies that may simulate myocardial infarction (MI), Brugada syndrome, or phenotypic manifestations of both hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy (Figure 51-3). In one study of nearly 2000 American football players (67% black), 5.8% of black players had an ECG appearance that was deemed “markedly abnormal” by the investigators, compared with only 1.8% of white players.26 Only a small proportion underwent subsequent echocardiographic evaluation, but the investigators concluded that in the majority of cases, the changes observed represented a normal ethnic variation.
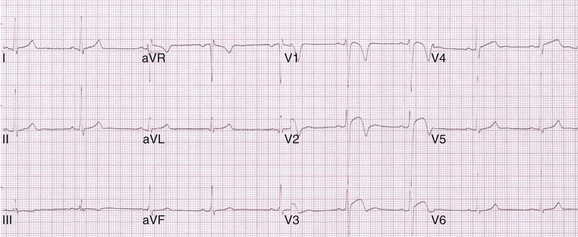
Our experience of evaluating large numbers of adolescent and adult male athletes of Afro-Caribbean origin has revealed that 25% exhibit marked repolarization changes comprising convex ST-segment elevation and deep (≥0.2 mV) T-wave inversions in leads V1 to V4 (Figure 51-4).27 Such anomalies do not correlate with cardiac hypertrophy, chamber enlargement, or cardiac arrhythmias during exercise testing and on 24-hour ambulatory Holter recordings. Published data pertaining to female black athletes indicate that 14% of adult black female athletes exhibit minor T-wave inversions in the anterior precordial leads compared with less than 1% of white female athletes in the absence of structural heart disease (Figure 51-5).28 The precise mechanism and significance of such anomalies remains to be elucidated and requires longitudinal assessment.
Sporting Discipline
Sporting discipline affects cardiac adaptation. In general, athletes participating in purely endurance sports such as cycling, rowing, and long-distance running exhibit larger left ventricular cavities and a greater magnitude of left ventricular hypertrophy compared with athletes involved in purely isometric sports, such as wrestling and judo. Athletes participating in long-distance endurance sports are more likely to exhibit sinus bradycardia, voltage criteria for cardiac chamber enlargement, and repolarization changes.29 We advise caution against such generalizations because the training programs involved in most sports, particularly for athletes participating at the regional or national level, are arduous, physically intensive, and involve a combination of isotonic and isometric exercises. In our experience, an enormous overlap in ECG patterns exists among athletes participating in a large range of ball, racket, endurance, and power sports.
Uncommon Electrocardiogram Findings in White Athletes
T-Wave Inversions
Repolarization changes consisting of ST-segment depression and T-wave inversions are much less frequent than ST-segment elevation and high-voltage T waves, being present in 3% to 4% of white adolescent and adult athletes.11,23 The prevalence of T-wave inversions is significantly lower in white female athletes and considerably higher in male and female black athletes. These electrical anomalies overlap with the phenotypic manifestations of cardiomyopathy, ion channel disorders, and anabolic steroid abuse (Table 51-3) and warrant thorough evaluation before being attributed to physiological adaptation.30–33 In a longitudinal study of 123 adult male white athletes with deep T-wave inversions, 39 (32%) had echocardiographic and clinical evidence of cardiac disease and were treated appropriately for their respective conditions.34 Of the 84 remaining athletes without demonstrable cardiac disease, 81 were followed up for a mean of 9 years (±7; range, 1 to 27 years). Of these, 5 athletes (6%) subsequently developed a cardiomyopathy, with one death and one aborted cardiac arrest as a consequence of ventricular fibrillation (VF) caused by arrhythmogenic right ventricular cardiomyopathy and hypertrophic cardiomyopathy, respectively. The remaining 70 athletes did not develop any pathologic cardiac conditions during the period of follow-up. These data suggest that the presence of deep T-wave inversions in an athlete may be a harbinger of subtle cardiomyopathies and the risk of fatal ventricular arrhythmias and that such athletes should remain under periodic surveillance. T-wave inversion patterns in the anterior precordial leads (V1 to V3/V4) may be acceptable in adolescent athletes and in those of Afro-Caribbean origin; however, T-wave inversions in the lateral leads in adolescent athletes or black athletes warrant further evaluation for cardiac disease.27
Table 51-3 ECG Changes in a Number of Cardiac Diseases That May Be Observed During Pre-participation Screening
| CARDIAC DISEASE | ECG ABNORMALITIES |
|---|---|
| Arrhythmogenic right ventricular cardiomyopathy | Anterior T-wave inversions |
| Incomplete or complete RBBB | |
| ε-waves | |
| Hypertrophic cardiomyopathy | Left ventricular hypertrophy |
| Pathologic Q waves | |
| T-wave inversions—anterior or inferior | |
| ST-segment depression | |
| Rarely normal | |
| Idiopathic dilated cardiomyopathy | Intraventricular conduction abnormalities |
| Long QT syndrome | Prolonged Q-T interval |
| Brugada syndrome | Incomplete or complete RBBB |
| Anterior ST elevation | |
| Anomalous coronary arteries | Normal |
| Coronary artery disease | Usually normal |
| Wolff-Parkinson-White syndrome | Short P-R interval |
| δ-waves | |
| Anabolic steroid abuse | Q-T interval prolongation |
| T-wave inversions |
ECG, Electrocardiogram; RBBB, right bundle branch block.
Prolonged Q-T Interval
A long Q-T interval (>440 ms in males and >450 ms in females) is identified in 0.4% to 0.69% of athletes and is an indication for disqualification from competitive sport to minimize the risk of SCD, according to the European Society of Sports Cardiology guidelines.13,35 However, the prevalence of a long Q-T interval in athletes is significantly higher than the actual prevalence of congenital long QT syndrome (LQTS) (0.04%), possibly because the Q-T interval may be overestimated by Bazzet’s formula at heart rates below 40 beats/min and measurements may also be influenced by sinus arrhythmia and slightly wider QRS complexes observed in athletes compared with the general population.36 The positive predictive value (PPV) of an isolated corrected Q-T interval in the 440- to 470-ms range is not precisely known but is likely to be low, probably less than 10%. On the basis of these considerations, the Bethesda guidelines recommend an upper QTc limit of 470 ms in males and 480 ms in females.14 Our experience of athletes with long Q-T intervals suggests that a QTc 500 ms or greater is highly suggestive of LQTS, even in the absence of symptoms or an overt family history, and is usually associated with the other features of the disorder on exercise stress testing, such as paradoxical prolongation of the Q-T interval with increasing heart rate (up to 120 beats/min) and a long Q-T interval in other first-degree relatives.35
Overlap with Disease: Athlete’s Heart or Cardiomyopathy?
Hypertrophic Cardiomyopathy
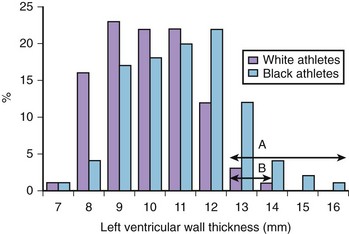
Cardiac adaptation to exercise is associated with modest increases in cardiac chamber wall thickness and cavity size. Typically, the left ventricular wall thickness in increased by 15% to 20%, and left ventricular cavity size may be increased by 10%.16 Occasionally, some male athletes (1.5% to 3% of white and 18% black athletes) (Figure 51-6) exhibit substantial increases in left ventricular wall thickness ranging from 13 mm to 16 mm, which fall within values observed in individuals with morphologically mild hypertrophic cardiomyopathy, which is the commonest cause of death in the young (age <35 years) worldwide (see Table 51-1).37–40 The distinction between the athlete’s heart and hypertrophic cardiomyopathy is vital because an erroneous diagnosis has the potential for serious consequences (see Table 51-3). The differentiation between the two entities is possible with the assessment of cardiac chamber size and indices of systolic and diastolic functions using echocardiography and cardiac magnetic resonance imaging (MRI); abnormalities on the 12-lead ECG may provide crucial information to unravel the clinical dilemma, which may prove challenging.41–43 The presence of deep T-wave inversions in any lead other than V1 in white athletes and in leads other than V1 to V4 in black athletes, pathologic Q waves, ST-segment depression, and left bundle branch block (LBBB) indicates pathologic left ventricular hypertrophy (Figure 51-7). Conversely, identification of the Sokolow-Lyon voltage criterion for left ventricular hypertrophy in isolation (without other associated features of left ventricular hypertrophy, including ST-segment depression, T-wave inversions, leftward axis, and voltage criterion for left atrial enlargement) is highly suggestive of physiological left ventricular hypertrophy (Figure 51-8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree