The name “Eisenmenger” refers to Victor Eisenmenger, an Austrian physician who in 1897 described the clinical and pathologic features in a patient with a large ventricular septal defect (VSD). Dr. Eisenmenger described a “powerfully built man of 32 years” in whom cyanosis increased considerably on effort. The patient’s clinical course changed to one of cyanosis, clubbing, and a slow progressive decline in functional ability. This was followed by dyspnea and edema with a state of “heart failure.” His clinical exam revealed elevated venous pressure, liver distention, and extensive edema. The patient collapsed and died following a large hemoptysis. Autopsy revealed a 2.5-cm membranous VSD with pulmonary but not aortic atherosclerosis. This case demonstrates many salient points for the purpose of this chapter.
■Adult survival
■Pulmonary arteriopathy that includes changes similar to atherosclerosis
■Variable cyanosis, increasing with exercise
■Secondary erythrocytosis (white cells are low normal, platelets are low)
■Hemoptysis
■Thrombosis
■Late onset heart failure
■Sudden death
Eisenmenger syndrome (ES), as defined by Paul Wood in 1958, refers to any cardiac defect with initial left-to-right shunting that results in the development of pulmonary vascular changes with increased resistance and pulmonary hypertension. Initially one would think that this group would represent a very small minority of adult congenital heart cases and hopefully one day with early detection and correction, it will. But at the present time these patients comprise between 3% and 10% of patients seen at adult congenital heart disease clinics and as a group have one of the higher percentages of morbidity and mortality. ES was the largest group, representing 19% with sudden cardiac death in a recent report by Koyak that included 25,790 patients from the clinic databases of Canada, Belgium, and the Netherlands. Several factors, including ventricular dysfunction were associated with an increased incidence of sudden cardiac death. The other factors included supraventricular tachycardia, increased QRS duration, and QRS dispersion. But assessment of ventricular function remains the most challenging. The modality of assessment needs to be able to assess both the right and left ventricle, detect changes before clinical deterioration, and potentially show improvement with treatment modalities such as vasodilators. The right ventricle has been challenging to assess, especially the hypertensive or systemic RV. Echocardiography is the main modality for ventricular assessment.
This chapter applies to a group of congenital heart defects in which the development of systemic level pulmonary artery pressures is due to one or more nonrestrictive communications at the intracardiac or great vessel level. These can be classified as pre- or post-tricuspid valve and are outlined in Table 42.1. This classification has prognostic significance as well as containing important distinctions when using echocardiography for assessment. Van De Bruaene noted the difference in these two groups in a study of 58 patients with Eisenmenger syndrome. The pre-tricuspid group was older, had larger biatrial size as well as larger right ventricles. The post-tricuspid defects do not develop the right ventricular and pulmonary artery dilation. The reason for this is the onset of the volume and pressure overload. Nonrestrictive post-tricuspid defects result in immediate postnatal left-to-right shunting with both pressure and volume overload to the pulmonary vasculature. This results in the retention of features of the fetal heart, especially the right ventricle. It is the early adaptation of right ventricular function that contributes to the significantly improved outcome for this group of patients compared to all other forms of pulmonary hypertension.
When the defect is pre-tricuspid, the early stage is only volume overload on the pulmonary vasculature. This does not take place during infancy or childhood and may not do so for several years or decades. A nonrestrictive ASD of 2–3 cm in size with normal left ventricular compliance will not develop a significant left-to-right shunt (Qp/Qs >1.5:1) until LV compliance declines. This explains why the exam of an infant or child with a nonrestrictive atrial septal defect may not have the usual findings of increased pulmonary flow, including the fixed split second heart sound and pulmonary artery systolic flow murmur. As left ventricular compliance declines, the shunt increases and commonly exceeds a Qp/Qs of 3:1. With normal Qs of 4–5 L/min, the right ventricle and pulmonary artery are exposed to a Qp of 12–15 L/min. Again, this volume overload most commonly does not happen for several decades. It will then take several years to decades for this volume to trigger the hypertensive pulmonary arteriopathy changes that result in pressure overload.
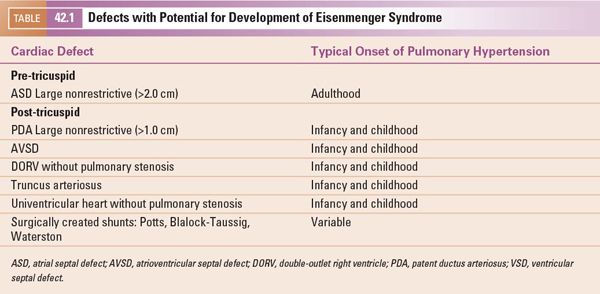
Thus, the pre-tricuspid defects allow the transformation of the fetal heart to the normal heart in infancy. This normal infant heart includes low pulmonary vascular resistance and pressure, as well as thinning of the right ventricular free wall to 3–5 mm with the normal septal configuration. This normal heart can then show the adaptive features to the low pressure, increased volume state associated with a pre-tricuspid defect. The post-tricuspid defect retains the crescent-shaped RV and LV size with the septum flat and midline. These are demonstrated in Figure 42.1. Figure 42.2 shows the adaptive changes of the RV and PA to the longstanding volume overload and subsequent pressure overload as seen in ES. The RV and PA can become massively dilated. Right heart failure can develop as well as pulmonary artery thrombosis. These topics will be covered in subsequent sections of this chapter.
The reaction in the pulmonary vasculature called the Eisenmenger “reaction” by Hopkins results in pathologic changes called hypertensive pulmonary arteriopathy. These develop to alleviate the volume-overloaded pulmonary vasculature and resultant heart failure. The changes are progressive, become nonreversible and result in pulmonary pressures equal to systemic pressures with little to no ability to be lowered even when the physiologic demand would warrant it. The degree of pulmonary hypertension is severe enough to cause the reversal of shunting and subsequent systemic desaturation and central cyanosis. The anatomy and physiology of lesions which may develop pulmonary hypertension are covered elsewhere in this text. In addition, key chapters review echocardiographic tools in the assessment of pulmonary hypertension. This chapter will focus on the adult patient with Eisenmenger syndrome and expand upon those chapters dealing with specific problems in this group of patients.
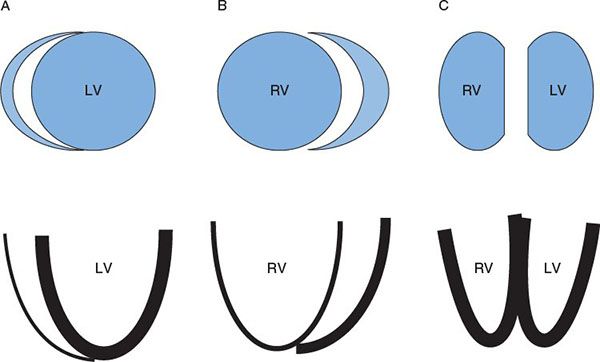
Figure 42.1. The top is a short-axis view of RV and LV chamber configuration. A: Normal. B: Pre-tricuspid defect with volume overload without pulmonary hypertension. C: Post-tricuspid defect with pressure and volume overload. The bottom is the ventricular wall configuration with line thickness representing wall thickness. RV, right ventricle; LV, left ventricle.
Figure 42.2. Massive MPA dilation in an adult with Eisenmenger syndrome. This patient developed a thrombus on the posterior aspect of the proximal right pulmonary artery.
ANATOMIC AND HEMODYNAMIC FINDINGS
Adults with Eisenmenger syndrome, regardless of the cardiac defect, have similar pulmonary vascular pathology. The pathophysiologic mechanisms contributing to these changes include shear stress, flow, pressure, thrombosis, and inflammation. Heath and Edwards first described the grading of the changes. The sequence of these changes subsequently has been shown not to progress from stage I through VI but rather to have an inflammatory component as described in grade VI that occurs before grades IV and V. The pathologic changes result in elevated pulmonary vascular resistance (Fig. 42.3).
It is this abnormality and the presence of a systemic-to-pulmonary connection that result in the features of Eisenmenger syndrome. All patients will have central cyanosis, clubbing, and physical examination findings of pulmonary hypertension with a parasternal lift and loud P2 component of the second heart sound. However, without myocardial dysfunction, they should not have the murmur of a significant left-to-right shunt. In fact they are generally without murmur but may have mild pulmonary insufficiency, and a prominent jugular venous “a” wave. The typical echocardiographic findings are demonstrated in Figures 42.4 and 42.5. All patients will have systemic pressures in the pulmonary artery and right ventricle and their defect will be nonrestrictive, without a large gradient.
ROLE OF ECHOCARDIOGRAPHY
Echocardiography is the cornerstone imaging modality in the evaluation of patients with Eisenmenger syndrome and it is crucial in the evaluation of endocarditis, valvular disease, thrombosis, and ventricular dysfunction. Since the first edition of this textbook, there have been further developments in the assessment of ventricular performance before pump failure, which may aid in the detection and potential treatment of these patients. We will first describe the features of the fetal right ventricle and the transformation with normal anatomy and correlate this with echocardiographic assessment. During fetal development the right and left heart circulations have equal pressures throughout the cardiac cycle. The fetal right heart characteristics are outlined in Table 42.2 and are compared to normal and Eisenmenger syndrome.
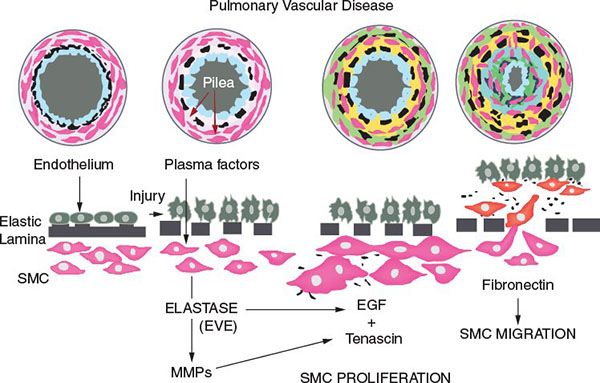
Figure 42.3. Inflammatory process associated with smooth muscle cell (SMC) proliferation in pulmonary vascular obstructive disease. (Courtesy of Dr. Marlene Rabinovich.)
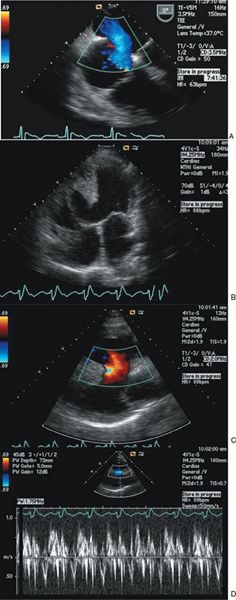
Figure 42.4. A: Transesophageal echocardiographic images demonstrating a large secundum atrial septal defect (ASD) with a Qp/Qs value of 1.8 and severe pulmonary hypertension. Left-to-right shunting during ventricular systole. B to D: Transthoracic echocardiography images of a large nonrestrictive ventricular septal defect (VSD) in an adult patient with double-outlet right ventricle. B: Two-dimensional image demonstrating large VSD. C: Laminar color Doppler flow representing left-to-right shunt through the VSD during systole. D: Spectral pulsed-wave Doppler sampled in the VSD showing low-velocity bidirectional shunting.
ASSESSMENT OF VENTRICULAR FUNCTION IN EISENMENGER SYNDROME
Until recently, assessment of RV function was limited to qualitative analysis as well as some global quantitative assessments that were predominantly developed for the left ventricle, such as ejection fraction (EF) and fractional shortening (Fig. 42.6). Other assessment tools used modalities that evaluated the longitudinal work of the RV such as tricuspid annular plane systolic excursion (TAPSE). However, as can be seen in Table 42.2, the post-tricuspid defect hypertensive RV takes on more of a circumferential contraction pattern but without the wringing-type twist characteristics of the LV. Thus, assessment of ventricular performance of the RV and LV may require different tools.
In the largest series to date, Moceri described echocardiographic variables in 181 consecutive patients with ES. In this study there was a heterogeneous mixture of patients: 16% pre-tricuspid ES, 41% with Down syndrome, 67% with functional NYHA Class 3 or 4 symptoms, and 41% who were receiving advanced pulmonary vasodilator therapy (bosentan and/or sildenafil). Predictors of mortality included TAPSE, peak systolic velocity, myocardial performance, RA area, and RA/LA area. They found the RV and RA to be significantly larger in the pre-tricuspid group but had no difference in longitudinal RV systolic function as assessed by TAPSE. However, the RV fractional area change (FAC) and tissue Doppler myocardial acceleration during isovolumic contraction were lower in patients with pre-tricuspid shunts, suggesting possibly abnormal circumferential function in the pre-tricuspid group. They also reported a marked increase in mortality (30% in 3 years) when TAPSE was <15 mm. The authors suggested a score based upon 1 point for each variable:
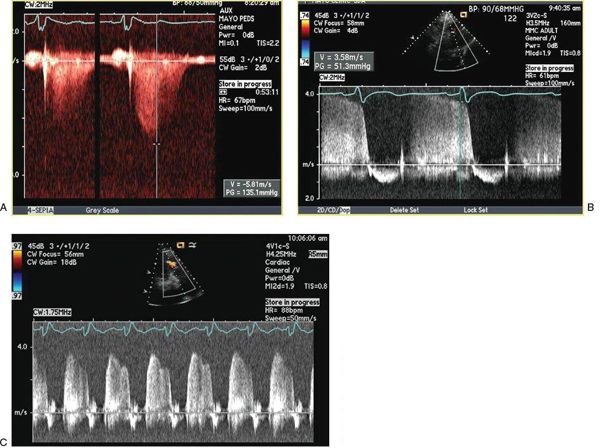
Figure 42.5. A, B: Continuous-wave spectral Doppler signals demonstrating typical tricuspid and pulmonary regurgitation velocity profiles obtained from an adult with Eisenmenger syndrome. C: Continuous-wave Doppler profile of pulmonary regurgitation demonstrating high early- and end-diastolic flow velocities consistent with pulmonary hypertension.
| TAPSE | <15 mm |
| Ratio of RV effective systolic to diastolic duration | ≥1.5 |
| RA area | ≥25 cm2 |



