The experience with the transradial approach in percutaneous coronary intervention (PCI) of chronic total occlusions (CTOs) in the United States is limited. We looked at the safety and feasibility of a home-made sheathless transradial technique (STT) with regular 8Fr catheters in CTO PCI. In March 2013, we developed an 8Fr STT for CTO PCI. We compared 119 patients who had the STT versus 122 treated with a standard transradial or transfemoral approach. The primary outcomes of interest were major vascular or bleeding site access complications. In a subgroup of patients with bilateral transradial approach, we assessed and compared radial patency 3 to 6 months after the procedure. Technical success rate of the CTO PCI was 93% in both groups. There were no major vascular or bleeding complications in the STT group. Radial hematomas were frequent but grade III occurred in 4 patients (3%) treated with the STT, not different to the incidence in the other group. The STT did not result in any increase in procedure time, contrast use, or radiation dose. Radial Doppler follow-up in 28 patients revealed 2 occlusions (7.1%) on the 8Fr shealthless side and one on the 6Fr side. In conclusion, our STT with regular 8Fr guides for CTO PCI is feasible, safe, and associated with low complication rate. We show that the hybrid CTO PCI nowadays can be performed through transradial access in the majority, with limited use of transfemoral approach.
We developed a sheathless transradial approach (TRA) with 8Fr catheters for chronic total occlusion (CTO) percutaneous coronary intervention (PCI). A standard 8Fr catheter with an external diameter of 2.67 mm corresponds to the external diameter of a 6Fr sheath. Therefore, our sheathless transradial technique (STT) with 8Fr catheter does not dilate the radial more than a 6Fr sheath does. From March 2013, we changed our approach in favor of 8Fr antegrade catheters, delivered preferentially with the STT. The present study compares patients treated with the STT versus those without STT, looking at success rate, contrast, radiation, and acute major vascular or bleeding complications. We subsequently assessed mid-term radial artery patency and diameters using Doppler in a subgroup of patients who underwent a bilateral TRA with a 6Fr retrograde and an 8Fr sheathless antegrade to further investigate the safety of the technique.
Methods
The Quebec Hybrid CTO PCI program started in January 2010, with the objective to maximize TRA for CTO PCI as much as for other PCIs in our institution. Initially, only 6Fr and rarely 7Fr guides were used. From March 2013, a decision was made to preferentially use an 8Fr antegrade catheter to facilitate the procedure, especially in the context of an increasing use of ADR techniques and the inability to perform the trapping balloon technique when ADR is performed through 6Fr. However, a TRA would still be preferred with the new STT as described later using 8Fr catheters. Otherwise, one femoral access would be used for the antegrade side. We kept using 6Fr catheters from the retrograde side in most cases. Baseline, procedural, and hospitalization data were prospectively collected and entered into a dedicated database. Data collection was approved by our institutional ethics committee as part of the Recherche Évaluative en Cardiologie InTervenionnelle registry and subjects signed informed consent for participation in the study. Those who underwent a bilateral TRA including one 8Fr STT and lived within 100 km from the institute signed a separate informed consent for Doppler follow-up.
The dilator of a 110-cm 6Fr Flexor Shuttle Sheath (Cook Inc., Bloomington, Indiana) (option 1) or, more recently, a 125-cm 6.5Fr Shuttle Select Slip-Cath carotid catheter (Cook Inc., Bloomington, Indiana) (option 2; Figure 1 ) is inserted into a 100-cm 8Fr Vista Brite Tip guide catheter (Cordis Corporation, Miami, Florida). Both catheters serve as if the operator would be introducing a long 6Fr sheath, but instead, it is the 8Fr catheter ( Figure 1 ). The third component of the system is a “bail-out” short 8Fr introducer sheath inserted around the catheter and pulled to the proximal hub. This sheath will only be inserted few centimeter into the radial artery in the case of oozing with the 8Fr catheter, which happens sometimes in patients with very large radials.

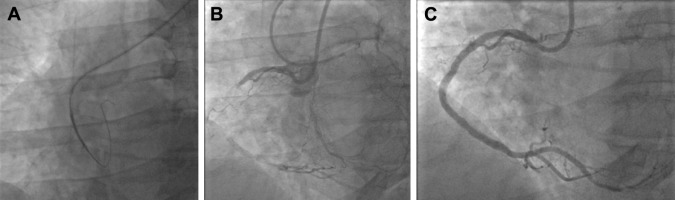
After gaining radial access as per usual techniques, we first insert a 6Fr introducer sheath in the radial artery and administer diltiazem 5 mg. After insertion of a standard 0.035′ guidewire into the introducer sheath, we remove the latter and open the skin entry site with a surgical blade while compressing the access site. Then, we insert the sheathless system. The tip of the Slip-Cath carotid catheter (or Flexor Shuttle Sheath dilator) tapers down nicely to the 0.035′ guidewire and is easily inserted on the wire into the radial. Then, the tip of the 8Fr guide follows. The full system is advanced through the radial to the ascending aorta ( Figure 2 ). After removal of the dilator or Slip-Cath, the catheter is engaged into the coronary ostium ( Figure 2 ).
Our patient selection criteria for STT were as follows: (1) patent palmar arch protecting against hand ischemia in case of a radial occlusion and (2) large (qualitatively) radial on palpation. We excluded flush ostial CTOs with minimal landing zone for the guide catheter. In such cases, a TFA was preferred as the guide moves in synchrony with the coronary ostium with breathing. When a radial was perceived by the operator to be too small, a TFA was used to deliver the 8Fr catheter, or a smaller catheter was used.
The way we perform CTO PCI has been published elsewhere and is based on the CTO anatomy. Apart from the use of 8Fr sheathless catheter, the equipment used during those procedures was at the discretion of the operator and followed a published algorithm. The occluded segment was always stented with drug-eluting stents and post-dilated as needed ( Figure 2 ).
Since March 2013, 3 patients underwent CTO PCI with a 7.5Fr Eaucath sheathless catheter (Asahi Intecc, Nagoya, Japan) and 4 patients had a 7Fr antegrade catheter delivered with STT (5.5 F Slip-Cath), as described earlier. These cases were excluded from the analysis. We then divided the study cohort into 2 groups: with 8Fr STT and without the STT. Then in a sample of patients who had a bilateral TRA with the 8Fr STT on one side and a regular 6Fr sheath on the other, we compared long-term outcomes of those radial arteries.
We assessed technical success rate, contrast volume, radiation, and major vascular (pseudo-aneurysm, arterial-venous fistula, thrombosis, compartment syndrome) or bleeding site access complications. The latter was defined by decrease of hemoglobin >3 g/L, associated with a retroperitoneal bleeding or a hematoma, as per the Bleeding Academic Research Consortium (BARC) definition. We evaluated the incidence of radial hematomas and considered important hematomas >10 cm above the entry site (type III, IV, or V). We also assessed hemoglobin decrease from baseline to the lowest inhospital, as a surrogate for bleeding from the catheters or other sources. We did not assess radial patency at discharge. In a subgroup of bilateral TRA patients who consented for follow-up Doppler, we compared radial patency and diameters 2 cm above the entry point, on the 8Fr sheathless versus the 6Fr sheath side, 3 to 6 months after the CTO PCI procedure. We examined the radial artery coronal lumen size from one intimal border to the other in the anteroposterior and lateral axes. For the vessel size, we measured from one adventitial border to the other in the anteroposterior and lateral axes. Dopplers were performed by hospital radiology technicians and reviewed by radiologists. Both were blinded to the 8Fr sheathless side.
Binary variables were expressed as percentages and continuous variables as mean values and SD when normally distributed or median and 25% to 75% interquartile range otherwise. Pairwise comparisons were performed using the Fisher’s exact test for discrete data and t tests for continuous variables, after confirming a normal distribution. Pairwise t test comparisons are not only presented with p values but also as mean difference with the 95% CI when more informative in the text. In the subgroup of patients who underwent Doppler follow-up, we assumed independence between the 2 radials in a given patient. Therefore, such an assumption resulted in a sample size of 28 radials that received a 6Fr sheath and 28 radials with sheathless 8Fr. Again, pairwise comparisons were performed using the Fisher’s exact test for discrete data, and t tests for diameters. Otherwise, a Wilcoxon rank-sum test was used. All analyses were conducted using SAS, 9.3 (Cary, North Carolina).
Results
From March 2013 to December 2015, a total of 119 patients underwent the STT using an 8Fr antegrade guide, whereas 122 were treated with standard TRA or TFA ( Table 1 ). The sheathless 8Fr TRA was feasible in all the selected patients with the catheter placed in coronary ostium without any significant radial spasm. As expected with the case selection criteria, most patients with STT were males, whereas a larger proportion of females were managed with a TFA ( Table 1 ). Patients in the STT group were also younger and less likely to be diabetics, all surrogate markers of a larger pulse on clinical assessment before the intervention. Again, as per case selection, CTOs treated with the STT were less likely to be ostial in location. Otherwise, case complexity (post-CABG cases, the average J-CTO, and the proportion of very difficult (score ≥3) cases) was similar in both groups. Technical success of CTO PCI was high in both groups. The STT did not result in any increase in procedure time, contrast use, or radiation dose. The 8Fr “bail-out sheath” was inserted in 3 patients to gain hemostasis at the wrist level.
| Variable | No sheathless (N=122) | Sheathless (N=119) | p-value |
|---|---|---|---|
| Age (years) | 68±9 | 64±11 | 0.003 |
| Men | 79(65%) | 113(95%) | <0.0001 |
| Diabetes mellitus | 56(46%) | 40(35%) | 0.08 |
| Hypertension | 114(93%) | 108(92%) | 0.73 |
| Smoker | 12(11%) | 23(22%) | 0.03 |
| Body mass index (kg/m 2 ) | 29±5 | 30±6 | 0.06 |
| Previous myocardial infarction | 67(55%) | 68(57%) | 0.78 |
| Previous percutaneous coronary intervention | 89(73%) | 90(76%) | 0.63 |
| Previous coronary bypass | 61(50%) | 46(39%) | 0.08 |
| Left ventricular ejection fraction (%) | 53±12 | 56±10 | 0.02 |
| Indication | 0.98 | ||
| CCS 1-2 angina pectoris | 35(29%) | 38(32%) | |
| CCS 3-4 angina pectoris | 48(39%) | 47(39%) | |
| UAP/MI | 21(17%) | 19(16%) | |
| Other | 18(15%) | 15(13%) | |
| Target CTO | 0.99 | ||
| Left main | 5(4%) | 6(5%) | |
| Left anterior descending | 14(12%) | 18(16%) | |
| Left circumflex and obtuse marginal | 33(27%) | 19(16%) | |
| Right | 64(53%) | 68(59%) | |
| Diagonal branches | 5(4%) | 5(4%) | |
| Japanese-CTO score | 2.3±1.2 | 2.3±1.4 | 0.76 |
| Japanese-CTO ≥3 (very difficult) | 60(49%) | 57(48%) | 0.84 |
| Lesion length >20mm | 50(41%) | 51(43%) | 0.77 |
| Lesion length >40mm | 18(15%) | 16(13%) | 0.77 |
| Previous failure | 33(27%) | 35(29%) | 0.68 |
| In-stent occlusion | 17(14%) | 14(12%) | 0.61 |
| Ostial location | 33(27%) | 21(18%) | 0.08 |
| Bridging collaterals | 33(27%) | 40(34%) | 0.27 |
| Branch at proximal entry | 68(56%) | 71(60%) | 0.59 |
| Dual access | 111(91%) | 113(95%) | 0.23 |
| Accesses | <0.0001 | ||
| Single radial | 8(7%) | 7(6%) | |
| Single femoral | 3(2%) | 0(0%) | |
| Dual radial | 29(24%) | 85(71%) | |
| Dual femoral | 16(13%) | 0(0%) | |
| Radial and femoral | 66(54%) | 27(23%) | |
| Antegrade catheter 8F | 61(50%) | 119(100%) | <0.0001 |
| Retrograde catheter 6F | 105(95%) | 104(92%) | 0.44 |
| Retrograde approach | 63(52%) | 59(50%) | 0.75 |
| Antegrade dissection-reentry | 30(25%) | 36(30%) | 0.32 |
| Dissection and re-entry | 57(48%) | 69(60%) | 0.07 |
| Technical success | 114(93%) | 111(93%) | 0.96 |
| Air kerma (Gy) | 3.3±2.0 | 3.3±1.9 | 0.91 |
| Radiation dose (cGy/cm 2 ) | 20403±13402 | 21910±14086 | 0.39 |
| Fluroscopy time, min | 64±36 | 58±35 | 0.19 |
| Contrast, ml | 344±138 | 349±169 | 0.83 |
| Procedure duration | 154±74 | 144±73 | 0.30 |
| Major bleeding complication | 2(2%) | 0(0%) | 0.16 |
| Major vascular complication | 1(1%) | 0(0%) | 0.31 |
| MACE | 7(6%) | 8(7%) | 0.75 |
| Radial hematoma | 27(23%) | 23(21%) | 0.72 |
| Forearm hematoma extending ≥10cm of entry point (type >III) | 3(3%) | 4(3%) | 0.92 |
| Femoral hematoma | 11(9%) | 6(5%) | 0.27 |
| Post-PCI hemoglobin drop (g/L) | -10±9.7 | -9±8.8 | 0.42 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


