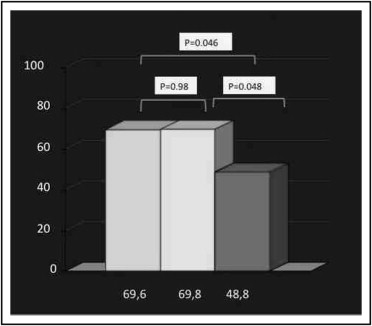
Bivalirudin is widely used as an anticoagulant during percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction. However, an increase in acute stent thrombosis rates has been found in the HORIZONS-AMI trial. A prolonged infusion after PCI has been shown to be a safe and effective tool in patients undergoing urgent or elective PCI in the PROBI VIRI study. We examined the effects of prolonged drug infusion after primary PCI. From databases of 5 high-volume centers we compared a group of patients treated with a 4-hour prolonged infusion after PCI to 2 groups treated with a peri-PCI infusion and heparin plus abciximab. The primary study end point was >70% ST-segment resolution within 90 minutes after PCI; secondary end points were partial (>50%) ST-segment resolution within 90 minutes and intrahospital major and minor bleedings on the Acuity scale. The study population consisted of 264 patients undergoing primary PCI who were pretreated with aspirin and clopidogrel. The 3 study groups did not differ significantly by baseline characteristics. The primary end point was achieved in 69.8%, 48.8%, and 69.6% of patients in the prolonged bivalirudin, bivalirudin, and heparin/abciximab groups, respectively (p = 0.048 for prolonged vs standard infusion, p = 0.98 for prolonged infusion vs abciximab). Major bleedings and other secondary study end points were not significantly different among study groups. In conclusion, a strategy of prolonged bivalirudin infusion after primary PCI seems equivalent to a strategy with heparin plus abciximab, with an improvement in standard infusion in obtaining early microvascular reperfusion.
Management of ST-segment elevation myocardial infarction (STEMI) in Western countries has achieved significant improvements mostly because of well-organized immediate management and transportation of patients and widespread use of primary percutaneous coronary intervention (PCI) focused on obtaining early vessel reopening. Antithrombotic treatment has a significant role in this improvement. However, if robust antiplatelet and antithrombin management increases bleeding risk, a “light” approach sometimes provides inadequate protection against recurrent ischemic events, and a balance between the 2 strategies is often difficult to achieve. Recently, the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial showed that a specific antithrombotic treatment with peri-PCI bivalirudin improves short- and medium-term survival compared to heparin plus glycoprotein IIb/IIIa inhibitors but at the expense of a higher acute stent thrombosis rate. We hypothesized that a postprocedure prolonged infusion of bivalirudin would warrant a better safety profile during the first hours after PCI as previously shown in other clinical settings.
Methods
The study population consisted of consecutive patients undergoing primary PCI who were enrolled in 5 Italian high-volume primary care hospitals from January 2009 through March 2010. From the hospital databases we compared 3 different study populations: patients treated similarly to those in the HORIZONS-AMI (peri-PCI only and bivalirudin), patients treated similarly to those in the PROlonged Bivalirudin Infusion Versus Intraprocedural only RandomIzed (PROBI VIRI) trial (peri-PCI and prolonged infusion of bivalirudin), and patients treated with standard care (unfractionated heparin [UFH] plus abciximab). To decrease precoronary time hospitals involved in this study use a direct-access facility for patients undergoing primary PCI who are transported directly from the field to the catheterization laboratory.
On admission to the catheterization laboratory all patients were treated with aspirin (500 mg intravenously) and clopidogrel (600 mg) and the drugs were used according to international guidelines. Study drug infusion was started just before angiography according to local practice. For UFH use an activated clotted time was measured every 30 minutes to maintain values from 200 to 250 seconds. UFH infusion was discontinued at the end of PCI. Abciximab was administered as an intravenous bolus (0.25 mg/kg) followed by a 12-hour infusion (0.125 μg/kg/min). Bivalirudin was administered as an intravenous bolus of 0.75 mg/kg and a 1.75-mg/kg/hour infusion for the duration of PCI (decreased to 0.25 mg/kg/hour for prolonged infusion).
PCI was performed according to international guidelines. Manual thrombus aspiration was attempted only for a high thrombotic burden at visual estimation. Bare-metal stent use was the first choice, whereas drug-eluting stents were reserved for diabetic patients with low thrombus burden. A 6Fr introducer was used in all patients. For a transradial route the introducer was removed just at the end of PCI; for a transfemoral route it was removed 3 hours after the intervention independently of treatment. On completion of PCI all patients had a 24-hour recording of cardiac rhythm with Holter monitoring to assess ST-segment resolution.
The primary study end point was complete (>70%) ST-segment resolution on electrocardiogram within 90 minutes after PCI. Secondary end points were partial (>50%) ST-segment resolution at 90 minutes, in-hospital major and minor bleedings (Acuity scale ), and 30-day and 9-month major adverse cardiac events, namely death, Q-wave MI, or target vessel revascularization.
Based on data in the literature we hypothesized incidences of 65% for the primary end point in the UFH/abciximab and prolonged bivalirudin groups and 55% in the peri-PCI only/bivalirudin group, a significantly lower incidence. Data are presented as mean ± SD for continuous variables and as absolute or relative frequency for categorical variables. Continuous variables were compared using paired t test and categorical variables using chi-square test.
Statistical significance and effect of treatment on outcomes were estimated with appropriate statistical methods for matched data. All reported p values are 2-sided and a p value of 0.05 was used as the cutoff for statistical significance. A multivariate logistic regression model was used to estimate odds ratios and 95% confidence intervals adjusting for gender, age, previous MI, anterior location of MI, cardiovascular risk factors, multivessel (MV) disease, thrombus aspiration attempt, and Thrombolysis In Myocardial Infarction grade 0 to 2 flow before intervention as potential confounding factors. Statistical analyses were performed using SPSS 13.0 (SPSS, Inc., Chicago, Illinois).
Results
The study population consisted of 264 patients who underwent primary PCI: 86 were treated with peri-PCI only/bivalirudin, 92 with UFH plus abciximab, and 86 with peri-PCI and 4-hour infusion of bivalirudin. Baseline clinical characteristics were well matched among study groups ( Table 1 ). Mean symptom-to-admission times were 92 ± 98 minutes, 88 ± 72 minutes, and 97 ± 82 minutes, respectively (p = 0.56). Mean door-to-drug and door-to-balloon times were also not significantly different among study groups. Abciximab was used in bailout fashion in only 6% of patients in the 2 bivalirudin arms because of a high thrombotic burden. Procedural data also were comparable among study groups ( Table 2 ).
| Variable | GPI (n = 92) | Prol Biv (n = 86) | Biv (n = 86) | p Value | ||
|---|---|---|---|---|---|---|
| GPI vs Prol Biv | GPI vs Biv | Biv vs Prol Biv | ||||
| Men | 68 (74%) | 60 (70%) | 58 (67%) | 0.61 | 0.56 | 0.87 |
| Age (years), mean ± SD | 64 ± 9 | 65 ± 10 | 63 ± 8 | 0.72 | 0.74 | 0.47 |
| Hypertension | 64 (70%) | 54 (63%) | 62 (72%) | 0.63 | 0.78 | 0.44 |
| Diabetes mellitus | 26 (28%) | 22 (26%) | 20 (23%) | 0.91 | 0.76 | 0.87 |
| Dyslipidemia | 54 (59%) | 38 (44%) | 44 (51%) | 0.41 | 0.76 | 0.44 |
| Previous revascularization | 12 (13%) | 10 (12%) | 10 (12%) | 0.96 | 0.96 | 1 |
| Previous myocardial infarction | 14 (15%) | 8 (9%) | 8 (9%) | 0.83 | 0.83 | 1 |
| Infarct territory | ||||||
| Anterior | 46 (50%) | 42 (49%) | 46 (53%) | 0.85 | 0.94 | 0.54 |
| Inferior | 38 (41%) | 40 (47%) | 32 (37%) | 0.79 | 0.54 | 0.47 |
| Other | 8 (9%) | 4 (5%) | 8 (9%) | 0.89 | 0.94 | 0.32 |
| Symptoms to administration (minutes), mean ± SD | 88 ± 72 | 97 ± 82 | 92 ± 98 | 0.54 | 0.79 | 0.54 |
| Administration to study drug (minutes), mean ± SD | 27 ± 12 | 32 ± 15 | 32 ± 17 | 0.46 | 0.48 | 0.93 |
| Door-to-balloon time (minutes), mean ± SD | 35 ± 14 | 39 ± 15 | 38 ± 16 | 0.64 | 0.59 | 0.87 |
| Variable | GPI (n = 92) | Prol Biv (n = 86) | Biv (n = 86) | p Value | ||
|---|---|---|---|---|---|---|
| GPI vs Prol Biv | GPI vs Biv | Biv vs Prol Biv | ||||
| Thrombus aspiration attempt | 82% | 79% | 76% | 0.54 | 0.43 | 0.47 |
| Radial access | 83% | 85% | 87% | 0.67 | 0.72 | 0.87 |
| Lesion length (mm), mean ± SD | 12 ± 6 | 13 ± 7 | 11 ± 7 | 0.76 | 0.54 | 0.43 |
| Thrombolysis In Myocardial Infarction grade 0–2 flow before percutaneous coronary intervention | 38% | 39% | 41% | 0.63 | 0.47 | 0.54 |
| Bailout glycoprotein IIb/IIIa inhibitor use | NA | 4% | 4% | — | — | 1 |
| Previous statin use | 54% | 49% | 56% | 0.67 | 0.76 | 0.43 |
| Previous angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use | 52% | 64% | 58% | 0.34 | 0.67 | 0.54 |
| Previous β-blocker use | 44% | 39% | 36% | 0.63 | 0.41 | 0.82 |
| Thrombolysis In Myocardial Infarction grade 0–2 flow after percutaneous coronary intervention | 2 | 2 | 3 | 0.89 | 0.76 | 0.81 |
| Myocardial blush grade 2–3 after percutaneous coronary intervention | 80 | 75 | 65 | 0.96 | 0.03 | 0.029 |
| Procedural success | 98% | 98% | 98% | 0.96 | 0.96 | 1 |
Complete ST-segment resolution after 90 minutes, the primary study end point, was improved in the 2 groups treated with prolonged bivalirudin infusion and UFH/abciximab compared to standard bivalirudin treatment (70%, 70%, and 49%, respectively, p = 0.048 for prolonged bivalirudin vs standard bivalirudin, p = 0.046 for UFH plus abciximab vs standard bivalirudin, p = 0.98 for prolonged bivalirudin vs UFH plus abciximab; Figure 1 ) . Myocardial reperfusion tout court also was improved in the abciximab and prolonged bivalirudin groups ( Figure 2 ) . The angiographic marker of microvascular reperfusion just after the intervention, the myocardial blush grade, was found to be improved in the UFH/abciximab and prolonged bivalirudin infusion groups compared to the normal bivalirudin group ( Table 2 ).