The safety of sirolimus-eluting stents (SESs) in acute myocardial infarction (AMI) remains controversial. We compared long-term neointimal coverage after stent implantation for AMI evaluated by coronary angioscopy and 3-year clinical events between SESs and bare-metal stents (BMSs). Eighty-seven consecutive patients who received SESs or BMSs for AMI were enrolled. At 8 months after AMI coronary angiography with angioscopy was performed. Using angioscopy we evaluated maximum and minimum grades of neointimal coverage using an angioscopic score (0 to 3). We calculated the heterogeneity score as the maximum grade minus the minimum grade. We compared angioscopic parameters including minimum grade and heterogeneity score of neointimal coverage, thrombi and plaque color, serum parameters, and major adverse cardiac events for 3 years between the 2 groups. The restenosis rate of the SES group (n = 56) was significantly lower than that of the BMS group (n = 31, 9% vs 31%, p = 0.015). The SES group had a lower minimum grade of neointimal coverage and higher heterogeneity score and prevalence of thrombi than the BMS group, but from 8 months to 3 years after stent implantation there were no significant differences in major adverse cardiac events between the 2 groups. In conclusion, a lower minimum grade and greater heterogeneity of neointimal coverage and thrombi were shown for SESs compared to BMSs at 8 months after AMI. However, these findings did not correlate with cardiac events over a period of 3 years in our patients.
Recent reports have identified a pathologic response of the vessel wall to drug-eluting stents (DESs) and delayed healing, which may serve as precursors to major adverse cardiac events (MACEs). Coronary angioscopy provides direct visualization of the lumen and detailed information on neointimal coverage beyond angiographic information. Although several angioscopic follow-up examinations after DES implantation have been reported, few angioscopic investigations comparing DESs to bare-metal stents (BMSs) for acute myocardial infarction (AMI) have been performed. In the present study we compared angioscopic findings, focusing on not only the minimum grade but also the heterogeneity of neointimal coverage, between sirolimus-eluting stents (SESs) and BMSs at 8 months after stent implantation for AMI; we also evaluated MACEs over a period of 3 years.
Methods
From October 2005 through August 2007 87 consecutive patients who underwent SES (Cypher, Cordis Corporation, Miami Lakes, Florida) or BMS (Driver, Medtronic Vascular, Santa Rosa, California) implantation for de novo lesions were enrolled in this study. Stent type, SES or BMS, was selected at the attending physicians’ discretion. Follow-up angiography with angioscopy was scheduled at 8 months after stent implantation in all patients. AMI was defined as chest pain of ≥30 minutes’ duration occurring within 6 hours of presentation, ST-segment elevation of ≥2 mm in 2 contiguous electrocardiographic leads, and a greater than threefold increase in serum creatine phosphokinase activities. Exclusion criteria included contraindications to antiplatelet therapy, renal dysfunction (serum creatinine >2.5 mg/dl) without hemodialysis, previous MI, and cardiogenic shock. The medical ethics committee at Osaka Rosai Hospital approved this study, and written informed consent was obtained from all patients before each catheterization.
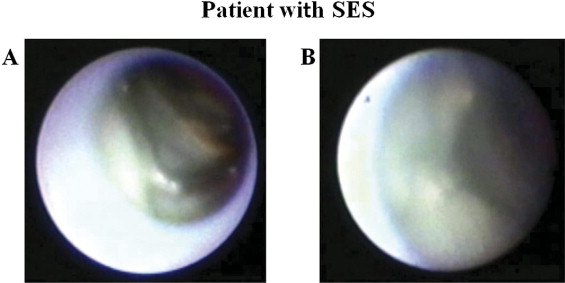
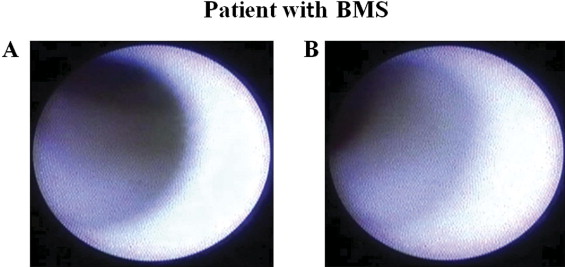
Coronary angiography was performed at 8 months after stent implantation by a radial approach using a 6Fr sheath just after administration of intravenous heparin (5,000 IU) and intracoronary isosorbide dinitrate (2.5 mg). In-stent restenosis was defined as >50% diameter stenosis by visual assessment. Angioscopy was performed in patients without in-stent restenosis. An FT-201 angioscope (Intertec Medicals Co., Ltd., Osaka, Japan) and an AS-003 optic fiber (Intertec Medical, Co., Ltd.) were used. Angioscopic observations were made after blood was cleared from the view, which was achieved by injection of 3% dextran through the probing catheter. Angioscopic images were recorded on digital video disks for subsequent analysis. Neointimal coverage in the present study was classified into the following 4 grades according to our previous report. Grade 0 represents stent struts that were exposed similarly to findings just after implantation; grade 1 represents stent struts with very thin neointimal coverage with no metallic luster; grade 2 represents stent struts that are embedded but visible; and grade 3 represents stent struts that are fully embedded and invisible. The grade of the best-covered segment was defined as the maximum grade, and the grade of the worst-covered segment was defined as the minimum grade. In this study the heterogeneity score was calculated as the maximum grade minus the minimum grade ( Figures 1 and 2 ) . We also evaluated the existence of thrombus and plaque color in the stent. Thrombus was defined as a coalescent red superficial or protruding mass that could not be flushed out by dextran solution injection, and plaque color in the stent was evaluated as yellow or white. Two independent observers who were unaware of patient and stent information analyzed angioscopic images to determine the inter-rater reliability of the angioscopic analysis.
We evaluated the following traditional coronary risk factors: co-morbidity of stroke and peripheral artery disease, medications, interventions, and serum parameters. Dyslipidemia was defined as treatment with medication or a serum low-density lipoprotein cholesterol level ≥140 mg/dl. Hypertension was defined as a blood pressure ≥150 mm Hg despite therapy for ≥3 months. Diabetes mellitus was defined current treatment of type 2 diabetes using hypoglycemic agents or a history of diabetes. Smoking was defined as ≥15 cigarettes/day. As prescribed medications we evaluated statins, anticoagulants, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, calcium antagonists, and β blockers. Administration of these medications began just after stenting and did not change during the follow-up period in any patient. As intervention parameters we evaluated lesion type (American College of Cardiology/American Heart Association type A/B1 or B2/C), length and diameter, and remodeling features using intravascular ultrasound (Atlantis Pro, Boston Scientific, Natick, Massachusetts) in all patients. Using intravascular ultrasound data we determined suitable stent size and length. After stent implantation we evaluated whether malapposition of the stent persisted at the edge of the stent by intravascular ultrasound. If malapposition of the stent was found, we performed postdilatation procedures at higher pressures until malapposition was alleviated. The remodeling index was defined as the vessel area at the site of the target lesion divided by the average vessel area of the reference segments proximal and distal to the target lesion. We defined positive remodeling as an index score >1.0. We also evaluated maximal inflation pressure for stent implantation, incidence of postdilatation procedures, and ratio of minimum stent area to proximal reference lumen area by intravascular ultrasound. As serum parameters we evaluated high-sensitive C-reactive protein, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting blood glucose, and hemoglobin A1c just before 8-month follow-up coronary angiography. As the other clinical parameters we evaluated the value of peak creatine phosphokinase, incidence of thrombectomy, and ejection fraction using the biplane Simpson method by echocardiography soon after AMI and just before 8-month follow-up coronary angiography.
Regular clinical follow-up visits occurred every month for up to 8 months after stent implantation. All patients with SES implantation received dual antiplatelet therapy with aspirin 100 mg/day and ticlopidine 200 mg/day for 1 year after SES implantation and then stopped receiving ticlopidine. All patients with BMS implantation received dual antiplatelet therapy using the same regimen for 2 months and then stopped receiving ticlopidine at 2 months after BMS implantation. All patients continued to receive aspirin throughout the follow-up period (3 years). After 8-month follow-up coronary angiography, clinical follow-up visits occurred every 2 months for up to 3 years after stent implantation in all patients. MACEs including AMI, target lesion revascularization, and cardiac death were evaluated during the 3-year follow-up period.
Statistical analysis was performed using StatView 5.0 MDSU (SAS Institute, Cary, North Carolina). Categorical variables are presented as percentages and were tested with chi-square statistics. Continuous variables are expressed as mean ± SD and were analyzed by Student’s t test. A p value <0.05 was considered statistically significant. Inter-rater reliability was evaluated by the Cohen kappa coefficient with values >0.80 considered to show almost perfect agreement.
Results
Incidence of restenosis with SESs was significantly lower than that with BMSs (9% vs 31%, p = 0.015). Other MACEs were not found in either group until 8-month follow-up coronary angiography. Excluding patients with restenosis, the study patients consisted of 51 patients treated with SESs and 22 treated with BMSs. On multiple stent deployment 2 overlapping stents were deployed in 3 patients in the SES group and 2 patients in the BMS group. Patient and lesion characteristics are listed in Table 1 . Coronary risk factors, prescribed medication, and serum parameters were also similar between the 2 groups ( Table 1 ).
| Variable | SES Group (n = 56) | BMS Group (n = 31) | p Value |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 69.3 ± 7.5 | 66.3 ± 6.4 | 0.095 |
| Men | 78.6% | 87.1% | 0.397 |
| Stroke | 1.8% | 0.0% | 0.454 |
| Peripheral artery disease | 16.1% | 25.8% | 0.397 |
| Dyslipidemia ⁎ | 50.0% | 54.3% | 0.372 |
| Hypertension † | 35.1% | 58.1% | 0.070 |
| Diabetes mellitus | 64.4% | 45.2% | 0.113 |
| Smoking | 32.1% | 29.0% | 0.813 |
| Medications | |||
| Statins | 85.7% | 87.1% | 0.858 |
| Anticoagulants | 12.5% | 9.6% | 0.693 |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 71.4% | 64.5% | 0.504 |
| Calcium antagonists | 35.7% | 45.2% | 0.322 |
| β Blockers | 89.3% | 87.1% | 0.759 |
| Coronary artery interventional markers | |||
| Left anterior descending coronary artery | 60.8% | 67.7% | 0.515 |
| Left circumflex coronary artery | 19.6% | 12.9% | 0.425 |
| Right coronary artery | 19.6% | 19.4% | 0.974 |
| American College of Cardiology/American Heart Association type A/B1 | 32.1 | 45.2 | 0.184 |
| American College of Cardiology/American Heart Association type B2/C | 67.9 | 54.8 | 0.184 |
| Lesion length (mm) | 20.9 ± 3.8 | 21.7 ± 4.6 | 0.462 |
| Lesion diameter (mm) | 3.1 ± 0.3 | 2.9 ± 0.3 | 0.130 |
| Positive remodeling | 62.5% | 64.5% | 0.852 |
| Overlapping stent | 5.4% | 6.5% | 0.834 |
| Stent area/proximal reference area | 1.0 ± 0.1 | 1.1 ± 0.2 | 0.294 |
| Maximum inflation pressure (atm) | 16.2 ± 2.6 | 15.8 ± 2.0 | 0.553 |
| Postdilatation procedures | 71.4% | 77.4% | 0.618 |
| Serum markers | |||
| High-sensitive C-reactive protein (mg/dl) | 0.99 ± 0.85 | 0.62 ± 0.80 | 0.092 |
| Low-density lipoprotein cholesterol (mg/dl) | 109.8 ± 29.1 | 107.5 ± 22.7 | 0.714 |
| High-density lipoprotein cholesterol (mg/dl) | 47.9 ± 9.9 | 47.1 ± 15.3 | 0.836 |
| Fasting blood glucose (mg/dl) | 115.0 ± 28.7 | 110.7 ± 27.1 | 0.545 |
| Hemoglobin A1c (%) | 5.9 ± 0.8 | 5.9 ± 1.0 | 0.948 |
⁎ Defined as treatment with medication or serum low-density lipoprotein cholesterol level ≥140 mg/dl.
† Defined as blood pressure ≥150 mm Hg despite therapy for ≥3 months.
Minimum grade of neointimal coverage in the BMS group was significantly higher than that in the SES group, whereas the heterogeneity score of neointimal coverage and incidence of angioscopic mural thrombus in the BMS group were significantly lower than those in the SES group. There were no significant differences in incidence of yellow plaque and AMI parameters including values of peak creatine phosphokinase, incidence of thrombectomy, and ejection fraction between the 2 groups ( Table 2 ). Cohen kappa coefficients for evaluation of inter-rater reliability were 0.88, 0.83, and 0.87 for minimum grade, maximum grade, and presence of mural thrombus, respectively. Representative cases are shown in Figures 1 and 2 .



