Inflammation predicts risk for cardiovascular disease (CVD) events, but the relation of drugs that directly target inflammation with CVD risk is not established. Methotrexate is a disease-modifying antirheumatic drug broadly used for the treatment of chronic inflammatory disorders. A systematic review and meta-analysis of evidence of relations of methotrexate with CVD occurrence were performed. Cohorts, case-control studies, and randomized trials were included if they reported associations between methotrexate and CVD risk. Inclusions and exclusions were independently adjudicated, and all data were extracted in duplicate. Pooled effects were calculated using inverse variance–weighted meta-analysis. Of 694 identified publications, 10 observational studies in which methotrexate was administered in patients with rheumatoid arthritis, psoriasis, or polyarthritis met the inclusion criteria. Methotrexate was associated with a 21% lower risk for total CVD (n = 10 studies, 95% confidence interval [CI] 0.73 to 0.87, p <0.001) and an 18% lower risk for myocardial infarction (n = 5, 95% CI 0.71 to 0.96, p = 0.01), without evidence for statistical between-study heterogeneity (p = 0.30 and p = 0.33, respectively). Among prespecified sources of heterogeneity explored, stronger associations were observed in studies that adjusted for underlying disease severity (relative risk 0.64, 95% CI 0.43 to 0.96, p <0.01) and for other concomitant medication (relative risk 0.73, 95% CI 0.63 to 0.84, p <0.001). Publication bias was potentially evident (funnel plot, Begg’s test, p = 0.06); excluding studies with extreme risk estimates did not, however, alter results (relative risk 0.81, 95% CI 0.74 to 0.89). In conclusion, methotrexate use is associated with a lower risk for CVD in patients with chronic inflammation. These findings suggest that a direct treatment of inflammation may reduce CVD risk.
Systemic inflammation is strongly linked to increased risk for cardiovascular disease (CVD). However, whether this relation is causal or simply an association is not established; no randomized trials have directly addressed whether targeted anti-inflammatory agents that do not have concomitant lipid-lowering or antiplatelet effects also reduce CVD event rates. Methotrexate has received particular interest in that regard, as it is a disease-modifying antirheumatic drug broadly used for the treatment of systemic inflammatory disorders, such as rheumatoid arthritis (RA) and psoriasis. Although the underlying mechanisms are not fully understood, methotrexate is known to ameliorate inflammatory responses by altering nucleotide metabolisms and, at least in part, mitigating cytokine signaling. The anti-inflammatory properties of methotrexate have been hypothesized to be beneficial in reducing CVD risk in patients with chronic inflammatory disorders (e.g., RA) or even in patients with persistent inflammatory responses (e.g., increased C-reactive protein levels). Recently, the evidence for relations between methotrexate use in patients with RA and CVD outcomes has been systematically reviewed. However, no systematic review and meta-analysis have been performed to critically and statistically evaluate heterogeneity among published studies in this field and to quantify the effects of methotrexate on CVD, which would help elucidate whether direct treatment of inflammation could potentially reduce CVD risk. To address this important question, we performed a systematic review and meta-analysis of the evidence for relations of methotrexate use with risk for CVD.
Methods
The Meta-Analysis of Observational Studies in Epidemiology guidelines were used as a reference for all stages of design, implementation, and reporting of this systematic review and meta-analysis.
We searched for all clinical trials or observational studies (prospective or retrospective or case-control studies) in which adults received methotrexate, the duration of follow-up was ≥3 months, and reported effect estimates on occurrence of “hard” CVD events (myocardial infarction [MI], coronary heart disease), sudden death, and/or stroke). We performed searches using multiple online databases, including MEDLINE (see “ Supplemental Methods ”), Embase, AGRIS, the Allied and Complementary Medicine Database, the Web of Knowledge, the Cumulative Index to Nursing and Allied Health Literature, CAB Abstracts, the Cochrane Library, conference abstracts (Zetoc), Faculty of 1000, and gray literature sources (System for Information on Grey Literature in Europe). We additionally reviewed related reports, hand-searched reference lists, and performed direct investigator contact. For each database, the years searched included the earliest available online year of indexing up to June 2010, without language restrictions. Key words were “methotrexate,” “mexate,” “amethopterin,” and “cardiovascular diseases.” We excluded a priori studies that had information only on intermediate secondary end points (e.g., lipid or glucose levels) or “soft” coronary heart disease outcomes (e.g., angina, heart failure), studies in which methotrexate was administered as part of a combination therapy, and studies in which users were very different from nonusers (e.g., diseased vs nondiseased participants). We also excluded a priori ecologic or cross-sectional studies; commentaries, general reviews, or case reports; duplicate publications from the same study; and studies reporting only crude risk estimates.
Of 694 identified reports, 621 were excluded on the basis of review of the title and abstract ( Figure 1 ) . The remaining 73 reports were reviewed in detail independently and in duplicate to determine inclusion or exclusion (95% concordance); differences were resolved by consensus or, if necessary, group consultation among all investigators. Sixty-three studies were excluded because they were reviews (n = 6), commentaries (n = 1), letters (n = 1), cross-sectional (n = 2), or in vitro (n = 1); included methotrexate users who were very different from nonusers (n = 1); assessed exposure other than methotrexate (n = 19); did not have appropriate control groups (n = 3); did not assess “hard” CVD end points (n = 27); or reported only crude risk estimates (n = 2) (see “ Supplemental Methods ”). In the end, 10 studies were included in this meta-analysis.
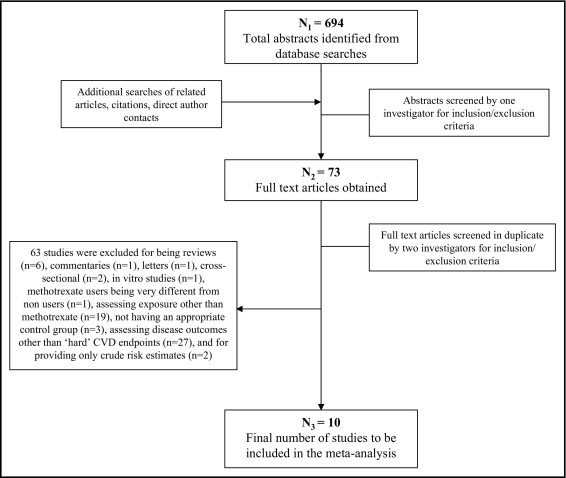
For each study, data were extracted independently and in duplicate by 2 investigators, including the year the study was published and performed, study location, study design, sample size and number of events, inclusion and exclusion criteria, duration of follow-up, underlying disease (e.g., RA), duration of underlying disease, assessment of underlying disease severity, methotrexate comparison groups (e.g., initiators vs noninitiators, ever users vs never users), ascertainment of methotrexate use, treatment dose, disease outcome, disease incidence versus recurrence, folate use, whether the reported analysis was primary or secondary and prespecified or post hoc in each report, covariates adjusted for in the analysis, and adjusted risk estimates and confidence intervals (CIs). Accepted standardized quality scores are not available for observational studies. We performed quality assessment as previously described and used, by evaluating and scoring 6 design criteria on an integer scale (0 or 1, with 1 being better), including review of study design and inclusion and exclusion criteria, assessment of exposure, assessment of outcome, control of confounding, assessment of underlying disease severity, and evidence of bias. These scores were summed, and quality scores from 0 to 3 were considered lower quality and scores from 4 to 6 higher quality. Differences in data extracted and quality assessment were very unusual and if present were resolved by group discussion and consensus. Missing data were obtained by direct investigator contact for only 1 of 10 studies, as described previously.
All included studies were observational and reported rate ratios or odds ratios; because of the low incidence of CVD in all studies, we collectively refer to these measures using the general term “relative risk” (RR). Between-study heterogeneity of RRs was assessed using the DerSimonian and Laird Q statistic, the I 2 statistic, and meta-regression. To calculate the overall pooled RR, we used a fixed-effects and a random-effects meta-analysis using the methods of DerSimonian and Laird and reported the former if the point estimates were virtually equal. In 1 study, methotrexate was considered as the reference group to estimate the RRs of other RA medications on risk for MI or stroke hospitalization, including biologics, other cytotoxic agents, noncytotoxic agents, and glucocorticoids. Subsequently, to estimate the RR of methotrexate compared to other RA medications (the latter as the reference group) on CVD risk, we pooled the inverse RRs of all other RA medications. Potential for publication bias was explored by visually inspecting a funnel plot of the effect size versus the SE and statistically using the Begg adjusted-rank correlation test. We explored potential prespecified sources of heterogeneity using stratified inverse variance–weighted fixed- and random-effects meta-analysis (and reported the latter if significant between-study heterogeneity was present, p <0.10) and inverse variance–weighted meta-regression, including study design (prospective vs retrospective cohorts), study location (America vs Europe), years of follow-up, methotrexate comparison groups (initiators vs noninitiators, ever vs never users, current vs noncurrent users), ascertainment of methotrexate use (physician vs self-reported), underlying disease (RA, psoriasis, polyarthritis), disease outcome (CVD, MI, stroke), event (incident vs recurrent), degree of covariate adjustment (most studies adjusted for sociodemographic characteristics and CVD risk factors, so further adjusting for underlying disease severity, other medication for underlying disease, or folate use), overall quality score (0 to 3 vs 4 to 6), and whether the reported analysis was primary or secondary and prespecified or post hoc (to address potential publication bias of “positive” findings) in each publication. Analyses were performed using Stata version 10.0 (StataCorp LP, College Station, Texas) with a 2-tailed α value <0.05.
Results
The 10 identified investigations included 8 prospective and 2 retrospective cohort studies in America (n = 6) and Europe (n = 4) and included 66,334 subjects in whom 6,235 CVD events were identified ( Table 1 ). We did not identify any randomized controlled trials that assigned methotrexate and assessed the occurrence of “hard” CVD events. One study provided 2 separate estimates for patients receiving methotrexate with RA and polyarthritis as the underlying disease (a total of 11 estimates). Therefore, 9 studies evaluated methotrexate use in RA as the underlying disease, 1 in psoriasis, and 1 in polyarthritis.
| Study | Country | Study Name | Underlying Disease | Ascertainment of MTX Use | MTX Comparison Groups | Disease Outcome | Disease Ascertainment | n | Events | Follow-Up (yrs) | Mean Age (yrs) | Adjustments ⁎ | Quality Score † |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective cohort studies | |||||||||||||
| Choi et al (2002) | United States | Wichita Arthritis Center Cohort | RA | Arthritis medical record database | Initiators vs noninitiators | CVD mortality § | Medical records, death certificates, or national death registry | 1,240 | 84 | 6.0 | 57 | 1, 2, 3, 4 | 6 |
| Solomon et al (2006) | United States | Pharmaceutical Assistance Contract for the Elderly | RA | Health care utilization database | Initiators vs noninitiators | MI or stroke hospitalization | Health care utilization database | 4,770 | 398 | 2.0 | 82 | 1, 2, 4 | 3 |
| MI hospitalization | 4,770 | Not reported | 2.0 | 82 | 1, 2, 4 | 3 | |||||||
| Stroke hospitalization | 4,770 | Not reported | 2.0 | 82 | 1, 2, 4 | 3 | |||||||
| Suissa et al (2006) | North America | PharMetrics Patient-Centric Outcomes Database Cohort | RA | Health care utilization database | Current users vs noncurrent users | AMI hospitalization ‡ | Medical records | 5,118 | 476 | 1.2 | 65 | 1, 2, 4 | 2 |
| Troelsen et al (2007) | Denmark | NA | RA | Clinical chart | Current users vs noncurrent users | IHD hospitalization § | Medical records, death and patients registry databases | 178 | 29 | 9.5 | 62 | 1, 2, 3 | 5 |
| MI hospitalization | 178 | 12 | 1, 2, 3 | 5 | |||||||||
| Nadareishvili et al (2008) | United States | National Data Bank for Rheumatic Disease Longitudinal Study | RA | Patients’ self-report | Ever users vs never users | Ischemic stroke ‡ | Medical records and death certificates | 832 | 41 | 4.0 | 70 | 2, 3 | 4 |
| Wolfe et al (2008) | United States | National Data Bank for Rheumatic Disease Longitudinal Study | RA | Patients’ self-report | Ever users vs never users | MI ‡ | Study questionnaires, medical records | 3,974 | 198 | 3.0 | 41 | 1, 2, 3 | 4 |
| Edwards et al (2008) | United Kingdom | United Kingdom General Practice Research Database | RA | General practice database | Ever users vs never users | MI ‡ | General practice database | 34,364 | 966 | 7 | 53.5 | 1, 2 | 1 |
| Goodson et al (2008) | United Kingdom | United Kingdom Norfolk Arthritis Register | Polyarthritis | Not reported | Current users vs never users | CVD mortality § | Not reported | 923 | 85 | 10.7 | 55 | 1, 2, 3, 4 | 3 |
| Retrospective cohort studies | |||||||||||||
| Prodanowich et al (2005) | United States | Miami Veterans Cohort | Psoriasis | Pharmacy database | Ever users vs never users | CVD ‡ | Medical records | 7,615 | 1,869 | Not reported ∥ | 65 | 1, 2, 5 | 2 |
| RA | 6,707 | 2,017 | Not reported ∥ | 66 | 1, 2, 5 | 2 | |||||||
| van Halm et al (2006) | The Netherlands | Jan van Breemen Institute | RA | Medical record | Ever users vs never users | CVD ‡ | Medical records | 613 | 72 | 9.2 | 64 | 1, 2, 3 | 3 |
⁎ Control of confounding: (1) sociodemographic indicators, (2) cardiovascular risk factors, (3) severity of underlying disease, (4) medications for underlying disease, (5) use of folate.
† Quality assessment was performed by review of study design, including inclusion and exclusion criteria, assessment of exposure, assessment of outcome, assessment of underlying disease severity, control of confounding (any 2 plus disease severity was given a score of 1; otherwise 0), and evidence of bias. Each of the 6 quality criteria was evaluated and scored on an integer scale (0 or 1, with 1 being better) and summed; quality scores ranging from 0 to 3 were considered lower quality and those ranging from 4 to 6 higher quality.
§ Not specified whether incident or recurrent event.
∥ Upon independent review by 4 investigators, duration of follow-up was believed to exceed 3 months, given the design and the number of incident events.
Five studies reported median duration of underlying disease, which ranged from 6 to 16 years. Most of the studies did not report dose of methotrexate treatment, and for those that did, the median dose ranged from 13 to 15 mg/week. With the exception of 1 study, all studies reported duration of follow-up; the median duration across studies was 5.84 years. In most of the studies, methotrexate use was ascertained by a physician (including prescription files and chart review); in only 2 studies, methotrexate use was self-reported. Half of the studies used health claims databases to ascertain methotrexate exposure. Most studies compared methotrexate ever users versus never users (n = 6 estimates), followed by current versus noncurrent users (n = 3 estimates) and initiators versus noninitiators (n = 2 estimates). Seven studies ascertained total or fatal CVD, 3 MI, and 1 ischemic stroke; 2 studies that evaluated CVD further provided separate estimates for MI and total stroke. Reported events were incident in 7 studies; case incidence versus recurrence was not specified for 3 studies. Quality scores were in the lower range (0 to 3) for most studies, reflecting mainly study design limitations, such as lack of control for potentially important confounders and lack of assessment of underlying disease severity. Degree of covariate adjustment also varied among studies; all studies adjusted for CVD risk factors (e.g., blood pressure, blood cholesterol, smoking), and all but 1 study adjusted for sociodemographic characteristics (e.g., age, gender, socioeconomic status, race); 6 adjusted for underlying disease severity, 4 for other medications used to treat the underlying disease, and 2 for folate use. For all studies, the reported exposure-outcome assessment was a prespecified primary or secondary aim.
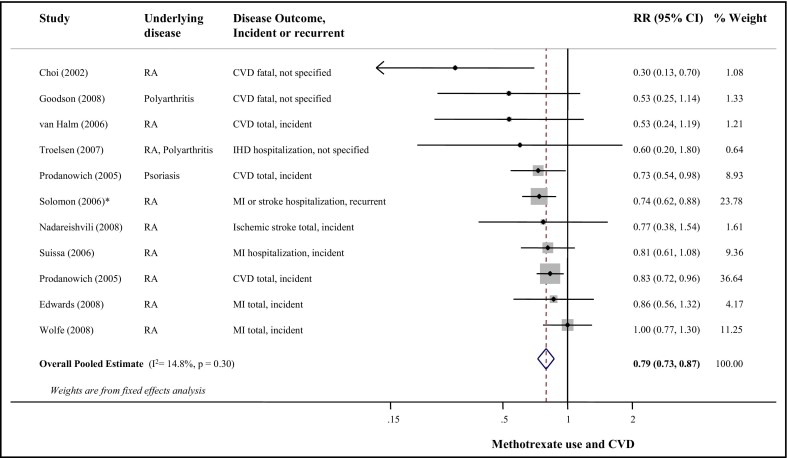
The p value for between-study heterogeneity was 0.30 (I 2 = 15%). Figure 2 presents the RR of CVD events associated with methotrexate use. Combining all studies, methotrexate use was associated with 21% lower CVD risk (95% CI 0.73 to 0.87); results of the random-effects meta-analysis were very similar (RR 0.79, 95% CI 0.71 to 0.88). Visual inspection of the funnel plot (see Supplemental Figure 1 ) suggested possible publication bias (p = 0.06), but excluding the 4 smallest studies with extreme risk estimates did not substantially alter the pooled estimate (RR 0.81, 95% CI 0.74 to 0.89). All p values for heterogeneity for potential prespecified sources were >0.14 in separate meta-regression models ( Table 2 ).
| Prespecified Source of Heterogeneity | Number of Estimates | Stratified Fixed-Effects Meta-Analysis RR (95% CI) | Meta-Regression p Value for Heterogeneity |
|---|---|---|---|
| Study design | |||
| Prospective cohort | 8 | 0.79 (0.70–0.89) | 0.97 |
| Retrospective cohort | 3 | 0.80 (0.70–0.91) | |
| Study location | |||
| America | 7 | 0.80 (0.73–0.88) | 0.51 |
| Europe | 4 | 0.71 (0.51–0.98) | |
| Duration of follow-up (years) | 9 | 0.79 (0.70–0.89) | 0.25 |
| MTX comparison groups | |||
| Initiators vs noninitiators | 2 | 0.52 (0.22–1.23) ⁎ | |
| Ever vs never users | 6 | 0.84 (0.75–0.93) | 0.22 |
| Current vs noncurrent users | 3 | 0.76 (0.59–0.99) | 0.70 |
| Ascertainment of MTX use | |||
| Physician | 8 | 0.78 (0.71–0.85) | 0.14 |
| Self-reported | 2 | 0.97 (0.76–1.24) | |
| Underlying disease | |||
| RA | 9 | 0.81 (0.73–0.88) | |
| Disease outcome | |||
| CVD (including IHD) | 7 | 0.76 (0.69–0.84) | |
| MI † | 3 | 0.82 (0.71–0.96) | 0.15 |
| Stroke † | 1 | 0.70 (0.56–0.87) | 0.94 |
| Event | |||
| Incident | 7 | 0.83 (0.75–0.92) | |
| Degree of covariate adjustment | |||
| Underlying disease severity | |||
| Yes | 6 | 0.64 (0.43–0.96) ⁎ | 0.88 |
| No | 5 | 0.79 (0.72–0.87) | |
| Other medication for underlying disease | |||
| Yes | 4 | 0.73 (0.63–0.84) | 0.22 |
| No | 7 | 0.83 (0.75–0.93) | |
| Folate use | |||
| Yes | 2 | 0.81 (0.71–0.92) | 0.83 |
| No | 9 | 0.78 (0.69–0.88) | |
| Quality score ‡ | |||
| Lower (0–3) | 7 | 0.78 (0.71–0.86) | 0.59 |
| Higher (4–6) | 4 | 0.68 (0.40–1.15) ⁎ | |
| Reported analysis | |||
| Primary | 6 | 0.78 (0.71–0.86) | 0.50 |
| Secondary | 5 | 0.87 (0.71–1.06) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


