Vascular complications are the most frequent adverse events associated with percutaneous coronary interventions (PCIs) leading to an increase in morbidity and mortality. Puncture of the common femoral artery in its middle segment is proved to decrease the risk of procedure-related vascular complications. Real-time ultrasound-guided puncture of the vessel is effective to decrease access site-related vascular complications but complex to perform. We evaluated whether an ultrasonic preinterventional examination of the femoral puncture site and skin marking of anatomic structures and specific vascular characteristics results in a decrease of access site-related vascular complications in PCIs with transfemoral access. Over a period of 12 months we prospectively examined all puncture sites before elective PCIs with transfemoral access (n = 848) using ultrasound. Presence, extent, and location of plaques and stenoses and exact location of bifurcation of the femoral artery were marked by a sonographer on the skin to guide the interventionists in vascular puncture. Postinterventional access site ultrasound was performed to determine possible access site-related complications. Frequency of vascular access site complications was compared to a control cohort (n = 1,027) that did not undergo ultrasound examination before intervention. With ultrasonic vascular access site management the rate of access site-related vascular complications was decreased from 4.2% to 1.9% (odds ratio 0.44, 0.23 to 0.80, p = 0.005). In conclusion, preinterventional ultrasonic access site examination and skin marking decreases the risk of vascular complications in elective PCI with femoral access.
The aim of this study was to evaluate whether a simple and rapidly performed preinterventional ultrasound examination of the femoral access site with skin marking of the preferred puncture site leads to a decrease of access site-related vascular complications.
Methods
In this single-center study all elective percutaneous coronary interventions (PCIs) performed with a transfemoral access from March 2008 through February 2009 were included. This relates to 848 access sites. Anticoagulation after sheath insertion was obtained using unfractionated heparin with a target activated clotting time of 250 to 300 seconds or 200 to 250 seconds if used in conjunction with glycoprotein IIb/IIIa inhibitor (GPI). Patients received GPI at the discretion of the treating physician according to standard protocol with abciximab or eptifibatide. Patients were given aspirin 100 mg/day and clopidogrel (300 or 600 mg as a loading dose followed by 75 mg/day) if stents were placed.
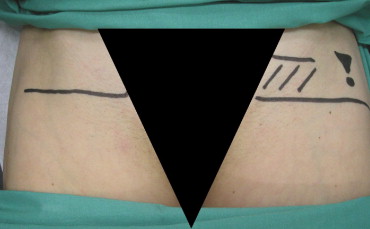
On the day before PCI all patients received clinical and ultrasound examinations of the groin and legs. Pulses of the femoral, popliteal, and tibial arteries were palpated and the groin auscultated. Subsequently an ultrasound examination of the groin was performed with measurement of the diameter of the common femoral artery and flow measurement by Doppler of the common femoral artery and proximal superficial femoral artery. The site of the femoral bifurcation and presence, extent, and location of plaques and stenosis were directly marked on the skin of the groin ( Figure 1 ) . In obese patients the bifurcation was marked laterally on the skin of the groin to avoid skin-marker displacement during intraoperative manipulation. Plaque presence in obese patients was indicated only by an exclamation point to warn the interventionist.
The control group included all patients undergoing elective PCIs in a 12-month period before performing the study. Pre- and postinterventional access site managements in this group were identical to the study group except for preinterventional ultrasound of the groin. Procedural techniques of PCI including antithrombotic therapy, sheath removal, and access site evaluation after the procedure were identical between the control and study groups.
During PCI localization of the puncture site was at the discretion of the operator. All operators were experienced interventional cardiologists with ≥300 arterial punctures annually. Sheath localization was documented by angiography. The recommended method of sheath removal was vascular closure device (VCD) deployment immediately after the procedure. The VCD Angio-Seal (St. Jude Medical, St. Paul, Minnesota) was the only VCD used during the entire study period. Patients did not undergo arterial closure with a VCD if (1) the arteriotomy site was ≤2 cm proximal to the bifurcation of the common femoral artery, below the femoral bifurcation, or above the inferior epigastric artery; (2) the common femoral artery had a vessel diameter ≤6 mm; (3) extensive calcification or plaque formation was present in the area of sheath insertion; and (4) extensive scar tissue was present at the access site. All operators had received training and had used the device in ≥50 cases before the study started. Sheath removal with manual compression (MC) took place in all patients with contraindications for VCD. MC sheath removal occurred when the activated clotting time was ≤150 seconds and a compression bandage was applied. Ambulation was initiated 4 hours after the VCD was placed and 6 hours after MC. Effective anticoagulation or administration of GPI was followed by 12-hour bandaging even if patients received a VCD.
Access site evaluation after the interventional procedure was carried out consistently in all patients in the 2 groups. Postinterventional assessment of the puncture site included clinical examination immediately after the catheterization procedure and after removal of the compression bandage. Examinations before the catheterization procedure and after removal of the compression bandage were performed by the same physician to ensure that changes were perceived. Any changes or abnormalities at the puncture site were reported to a vascular specialist resulting in vascular ultrasound and/or supplementary examinations such as computed tomography.
The following complications were recorded: bleeding defined as external blood loss requiring MC; oozing requiring light manual pressure; ecchymosis >5 cm in the soft tissue of the upper thigh; hematoma formation; retroperitoneal extension of hematoma, vessel obstruction, or closure; pseudoaneurysm; arteriovenous fistula confirmed by clinical examination, vascular ultrasonography, or computed tomography; infection of puncture site; decrease in hemoglobin concentration; need for blood transfusion; and need for surgery. Major vascular complications were defined as need for surgery, infection requiring intravenous antibiotics or surgical lancing, retroperitoneal hemorrhage, bleeding requiring blood transfusion, pseudoaneurysm, hematoma associated with a decrease in hemoglobin concentration by ≥30 g/L or ≥10% decrease in hematocrit, and postprocedural arteriovenous fistula.
Patient characteristics, anticoagulation medication (heparin, warfarin, GPI, aspirin, clopidogrel), and interventional procedure characteristics (sheath diameter, operator, additional sheaths, puncture site) were recorded in all patients.
All data were stored in a validated custom-made database. Datasets were inspected before analysis for outliers and inconsistencies. Continuous variables were checked for normality using the Shapiro–Wilke test. Continuous variables are described as mean ± SD and were compared using Student’s t test if normally distributed. Median and upper and lower quartiles are reported for non-normally distributed continuous variables and the nonparametric Wilcoxon rank-sum test was used. Categorical variables are presented as counts and percentages and Fisher’s exact test was used in all cases. A 2-sided p value of 0.05 was considered statistically significant. A generalized linear model for logistic regression was used for univariate and multivariate analyses. Variables with a p value ≤0.10 in univariate analyses were entered into multivariate models. Variables for univariate analysis including age, gender, weight, diabetes, hypertension, chronic renal insufficiency, anticoagulants, ultrasonic-guided puncture, use of VCD, and sheath size were predefined and selected on clinical grounds. Interaction terms were entered into the multivariate model based on clinical reasoning. Strata were built for continuous variables that were not normally distributed. In addition, all continues variables were dichotomized using the median as the cutoff unless a clinically defined cutoff was available (e.g., body mass index ≥25 kg/m 2 ). For final model selection a stepwise backward selection based on the model log-likelihood ratio was used. Statistical analyses were conducted using the R statistical programming language.
Results
During a period of 12 months 848 PCIs with a transfemoral access site were performed. Table 1 presents baseline characteristics of the study group (n = 848) compared to the control group (n = 1,027). Frequency of inappropriate punctures below the femoral bifurcation or above the inferior epigastric artery was decreased from 23.6% to 8.8% with ultrasonic-guided vascular access site management (odds ratio [OR] 0.31, 95% confidence interval [CI] 0.42 to 0.23, p <0.001) and, hence, the VCD application rate increased significantly from 68.7% to 75.1% (OR 1.37, 95% CI 1.12 to 1.70, p = 0.002). Major vascular complications were significantly decreased in the study group (1.9% in study group vs 4.2% in control group, OR 0.44, 95% CI 0.23 to 0.80, p = 0.005; Figure 2 ) . Table 2 presents the frequency of different access site-related vascular complications. Uni- and multivariate analyses identified use of preinterventional ultrasonography, use of VCD, gender, blood pressure, warfarin use, and use of GPI as independent predictors of access site-related vascular complications. No statistically significant interaction was present. Performance of preinterventional ultrasound and use of VCD were found to be associated with decreased risk of complications ( Table 3 ).
| Variable | Control Group | Study Group | p Value |
|---|---|---|---|
| (n = 1,027) | (n = 848) | ||
| Age (years) | 66.0 ± 10.9 | 67.7 ± 10.3 | <0.001 |
| Women | 26.8% | 24.9% | NS |
| Body mass index (kg/m 2 ) | 27.4 ± 4.2 | 28.6 ± 8.5 | <0.001 |
| Systolic blood pressure (mm Hg) | 142.7 ± 27.2 | 139.5 ± 24.8 | 0.01 |
| Diastolic blood pressure (mm Hg) | 68.1 ± 12.7 | 68.2 ± 13.7 | NS |
| Chronic renal insufficiency | 12.3% | 14.4% | NS |
| Diabetes mellitus | 38.2% | 37.4% | NS |
| Warfarin use | 11.2% | 12.9% | NS |
| Aspirin | 93.7% | 92.5% | NS |
| Clopidogrel | 93.6% | 99.9% | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 11.3% | 5.4% | <0.001 |
| Arterial sheath size ≥7Fr | 9.3% | 2.0% | <0.001 |
| Venous sheath | 23.2% | 20.8% | NS |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


