Prednisolone (PSL) therapy is the gold standard treatment in patients with cardiac sarcoidosis (CS). However, clinicians often have difficulty in deciding whether to discontinue PSL therapy in long-term management. Sixty-one consecutive patients with CS were divided into 2 groups based on the discontinuation of PSL during the median follow-up period of 9.9 years. PSL was discontinued in 12 patients because of improvement of clinical findings. There were no significant differences between the 2 groups in age, gender, left ventricular ejection fraction (LVEF), findings of imaging techniques, incidence of fatal arrhythmias and heart failure, and dose of PSL. After discontinuation of PSL, 5 patients had cardiac death, and discontinuation of PSL was significantly associated with higher cardiac mortality compared with continuation (p = 0.035). Although patients with discontinuation had improvement of LVEF after PSL treatment, LVEF decreased after discontinuation of PSL. Furthermore, discontinuation of PSL was associated with greater percent decrease in LVEF compared with continuation (p = 0.037) during the follow-up period. In conclusion, in the long-term management of patients with CS, discontinuation of PSL was associated with poor clinical outcomes and decreased LVEF, suggesting the importance of PSL maintenance therapy.
Despite a paucity of randomized clinical trial and no published clinical consensus guidelines with regard to the treatment of cardiac sarcoidosis (CS), prednisolone (PSL) therapy is the mainstay of treatment for CS to resolve active myocardial inflammation, which is related to the progression of granuloma development. Indeed, most previous studies suggest that PSL therapy may reduce fatal cardiac events with prevention of adverse left ventricular (LV) remodeling. However, there are few reports regarding the optimal treatment duration and timing of discontinuation of PSL therapy, especially in clinically stable patients showing no active myocardial inflammation detected by imaging techniques after PSL therapy. Hence, we set out to investigate the prognostic significance of discontinuing PSL therapy in newly diagnosed patients with CS treated with PSL.
Methods
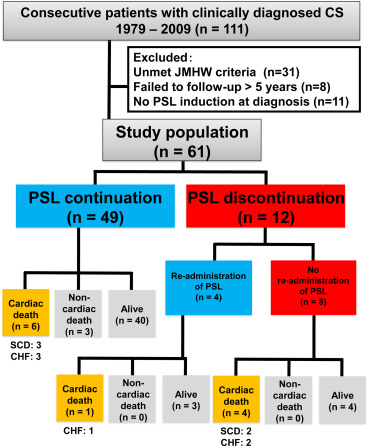
We examined 111 consecutive patients with newly suspected CS based on the clinical manifestations, without coronary artery disease, who were admitted to our institution from 1979 to 2009. Patients who failed to be followed for >5 years (n = 8), did not receive PSL at the time of diagnosis (n = 11), or did not meet the diagnostic criteria described in the 2006 revised version of the Japanese Ministry of Health and Welfare guidelines for CS (n = 31) were excluded. Finally, 61 patients were included in this study ( Figure 1 ). The study protocol agreed with the guidelines of the Ethics Committee of our institution (M25-047).
We collected the following data: age, gender, traditional coronary risk factors, cardiovascular medication, baseline fatal ventricular tachycardia (VT), advanced atrioventricular block (AVB), and congestive heart failure (CHF), extracardiac organ involvement, and findings of imaging techniques including gallium (Ga) scintigraphy, Fluorine-18-fluorodeoxy glucose-positron emission tomography (FDG-PET), and late gadolinium enhancement-cardiac magnetic resonance (LGE-CMR) at the time of diagnosis and within 6 months before discontinuation of PSL. Regarding FDG-PET findings, specific focal uptake of FDG was defined as positive based on previous reports. The presence of LGE in CMR was defined as any hyperenhancement in the myocardium. The findings of Ga, FDG-PET, and LGE-CMR were determined by the consensus of 2 experienced radiologists. Venous blood samples were serially obtained to measure plasma angiotensin-converting enzyme (ACE) activity, lysozyme level, hemoglobin, and serum creatinine and C-reactive protein levels.
Echocardiography was serially performed at the time of PSL induction, within 6 months before discontinuation of PSL, and at the end of the follow-up period. LV end-diastolic and end-systolic dimensions, thinning of the interventricular septum, and LV ejection fraction (LVEF) determined by the modified Simpson’s method were evaluated. All echocardiographic findings were interpreted by 2 experienced cardiologists.
The study end point was cardiac death, which was defined as sudden cardiac death (SCD), and death due to advanced CHF. Follow-up data were collected by direct contact with patients or patients’ physicians at the hospital or outpatient clinic, telephone interview of patients or, if deceased, of family members, and mail, by dedicated co-ordinators and investigators.
Results are presented as mean ± SD when normally distributed and as median and interquartile range (IQR) when non-normally distributed. Continuous variables were compared using paired or unpaired t test, or Mann-Whitney U test, when appropriate. Categorical variables are demonstrated as frequencies with percentages and were compared between the 2 groups using chi-square test or Fisher’s exact test. Long-term event-free survival was estimated using Kaplan-Meier curves, and log-rank (Mantel-Cox) test was used to assess differences according to the presence or absence of discontinuation of PSL during the follow-up period. All statistical analyses were performed with SPSS for Windows, version 21.0 (IBM Corp., Armonk, New York). Statistical significance was defined as a p value <0.05.
Results
During the median follow-up period of 9.9 years (IQR 7.9 to 13.0), PSL was discontinued in 12 patients (19.7%), and no patients with discontinuation had other immunosuppressants as an alternative to PSL. No significant differences were noted between the 2 groups with respect to age, gender, incidence of advanced AVB, VT, or CHF. The prevalence of traditional cardiovascular risk factors and extracardiac organ involvement with sarcoidosis were similar in the 2 groups ( Table 1 ).
| Variable | Overall (n = 61) | Prednisolone | P-value | |
|---|---|---|---|---|
| Continuation (n = 49) | Discontinuation (n = 12) | |||
| Age (years) | 59 (52,67) | 59 (54,68) | 57 (46,67) | 0.49 |
| Female | 44 (72 %) | 35 (71 %) | 9 (75 %) | 1.00 |
| Hypertension | 9 (15 %) | 9 (18 %) | 0 | 0.18 |
| Dyslipidemia | 16 (26 %) | 14 (29 %) | 2 (17 %) | 0.49 |
| Diabetes mellitus | 4 (7 %) | 4 (8 %) | 0 | 0.58 |
| Atrioventricular block | 18 (30 %) | 14 (29 %) | 4 (33 %) | 0.74 |
| Ventricular tachycardia / fibrillation | 22 (36 %) | 17 (35 %) | 5 (42 %) | 0.74 |
| Congestive heart failure | 9 (15 %) | 8 (16 %) | 1 (8 %) | 0.67 |
| Sarcoid granulomas in: | ||||
| Lung | 35 (57 %) | 27 (55 %) | 8 (67 %) | 0.53 |
| Skin | 9 (15 %) | 8 (16 %) | 1 (8 %) | 0.67 |
| Eye | 19 (31 %) | 18 (37 %) | 1 (8 %) | 0.083 |
| Number of involved organs | 2.0 (2.0,3.0) | 2.0 (2.0,3.0) | 2.0 (1.3, 2.0) | 0.22 |
| Medications | ||||
| Prednisolone induction dose (mg/day) | 30 (30,30) | 30 (30,30) | 30 (30,30) | 0.74 |
| Prednisolone minimum maintenance dose (mg/day) | 5 (5,10) | 5 (5,10) | 5 (5,10) | 0.41 |
| Other immunosuppressants | 1 (2 %) | 1 (2 %) | 0 | 1.00 |
| Angiotensin converting enzyme inhibitors | 20 (33 %) | 17 (35 %) | 3 (25 %) | 0.73 |
| Angiotensin receptor blockers | 10 (16 %) | 9 (18 %) | 1 (8 %) | 0.67 |
| Beta blockers | 26 (43 %) | 21 (43 %) | 5 (42 %) | 1.00 |
| Diuretics | 21 (34 %) | 17 (35 %) | 4 (33 %) | 1.00 |
The dose of PSL, the use of other immunosuppressants, and administration of cardiovascular medications, including ACE inhibitors and angiotensin receptor blockers, β blockers, and diuretics, were comparable between the 2 groups ( Table 1 ).
Findings of laboratory data and imaging techniques are listed in Table 2 . Laboratory data, including plasma ACE activity, lysozyme level, hemoglobin, and serum creatinine and C-reactive protein levels, were comparable between the 2 groups. Baseline LV end-diastolic dimension, end-systolic dimension, LVEF, and prevalence of basal interventricular septum thinning did not significantly differ between the 2 groups. The rates of positive findings in Ga scintigraphy, FDG-PET, and LGE-CMR imaging were comparable between the 2 groups.
| Variable | Overall (n = 61) | Prednisolone | P-value | |
|---|---|---|---|---|
| Continuation (n = 49) | Discontinuation (n = 12) | |||
| Hemoglobin (g/dL) | 13.4 ± 1.5 | 13.3 ± 1.4 | 13.6 ± 1.6 | 0.57 |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.42 |
| Angiotensin converting enzyme (IU/L) | 15.4 ± 8.0 | 15.5 ± 8.3 | 14.9 ± 7.2 | 0.84 |
| Lysozyme (IU/L) | 10.0 ± 4.1 | 9.5 ± 4.0 | 12.1 ± 4.1 | 0.073 |
| C-reactive protein (mg/dL) | 0.1 (0.1,0.3) | 0.1 (0.1,0.3) | 0.2 (0.1,0.3) | 0.17 |
| Echocardiography | ||||
| Left ventricular diastolic diameter (mm) | 54 (48,65) | 54 (48,64) | 53 (48,68) | 0.90 |
| Left ventricular systolic diameter (mm) | 42 (31,54) | 42 (31,54) | 42 (30,54) | 0.96 |
| Left ventricular ejection fraction (%) | 36 (26,50) | 35 (25,47) | 42 (28,59) | 0.077 |
| Basal interventricular septum thinning | 28 (47 %) | 22 (46 %) | 6 (50 %) | 0.80 |
| Other Imaging Modalities | ||||
| Gallium scintigraphy positive, positive / n | 38/58 (66 %) | 30/47 (64 %) | 8/11 (72 %) | 0.73 |
| 18 F-fluorodeoxy glucose-positron emission tomography positive, positive / n | 34/47 (72 %) | 26/38 (68 %) | 8/9 (89 %) | 0.41 |
| Late gadolinium enhancement-cardiac magnetic resonance positive, positive / n | 17/19 (89 %) | 16/18 (89 %) | 1/1 (100 %) | 1.00 |
The characteristics of the 12 patients in the PSL discontinuation group are summarized in Table 3 . In patients with discontinuation of PSL, 10 patients (83%) had positive inflammatory findings in imaging techniques. During the median PSL duration period of 2.4 years (IQR 1.2 to 4.0), only 1 patient (8%) developed AVB. The major reasons for PSL discontinuation were clinical improvement and/or negative inflammatory findings in imaging techniques after PSL maintenance; nevertheless, no patients developed serious side effects of PSL. Although 10 patients (83%) showed negative findings of inflammatory activity before PSL discontinuation, 8 patients had adverse events (3 VT/ventricular fibrillation, 2 AVB, 4 CHF, and 5 cardiac death) after PSL discontinuation, and PSL was re-administered in 4 patients with adverse events (50%).
| Case | Age (year) | Sex | Baseline Imaging (Findings) | Prednisolone Induction Dose, mg/day | Prednisolone Maintenance Dose, mg/day | Prednisolone Maintenance Period, year | Events Before Discontinuation | Reasons for Discontinuation | Imaging Before Discontinuation (Findings) | Events After Discontinuation | Re-administration of Prednisolone After Events |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alive | |||||||||||
| 1 | 50 | F | PET (positive) Gallium (negative) | 30 | 5 | 2.1 | – | PET (negative) No events | PET (negative) | – | – |
| 2 | 67 | F | PET (positive) Gallium (positive) | 30 | 2.5 | 3.5 | – | Gallium (negative) No events | Gallium (negative) | – | – |
| 3 | 30 | M | PET (negative) Gallium (positive) | 30 | 15 | 1.7 | – | PET (negative) Gallium (negative) No events | PET (negative) Gallium (negative) | Ventricular fibrillation | + |
| 4 | 45 | F | PET (positive) Gallium (positive) | 30 | 5 | 14.0 | – | PET (negative) No events | PET (negative) | – | – |
| 5 | 66 | F | Gallium (negative) | 30 | 5 | 1.2 | – | No events | Gallium (negative) | Ventricular tachycardia | + |
| 6 | 69 | F | PET (positive) Gallium (positive) | 30 | 15 | 0.4 | – | Atrio-ventricular block improvement | Gallium (positive) | Atrio-ventricular block Congestive heart failure | + |
| 7 | 59 | M | No data | 30 | 5 | 1.0 | – | No events | No data | – | – |
| Death | |||||||||||
| 8 | 66 | M | PET (positive) Gallium (positive) | 40 | 5 | 4.2 | – | Gallium (negative) No events | Gallium (negative) | Atrio-ventricular block Congestive heart failure | – |
| 9 | 72 | F | PET (positive) Gallium (positive) | 25 | 5 | 1.2 | – | Gallium (negative) No events | Gallium (negative) | Congestive heart failure | – |
| 10 | 50 | F | Gallium (positive) | 30 | 2.5 | 3.2 | – | Gallium (negative) No events | Gallium (negative) | Sudden cardiac death | |
| 11 | 39 | F | PET (positive) Gallium (positive) | 30 | 10 | 5.4 | Atrio-ventricular block | Gallium (negative) | Gallium (negative) | Ventricular tachycardia Congestive heart failure | + |
| 12 | 55 | F | PET (positive) Gallium (negative) | 30 | 5 | 2.8 | – | Gallium (negative) No events | Gallium (negative) | Sudden cardiac death | – |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


