Aortic valve morphology has been invoked as intrinsic to outcomes of balloon aortic valvuloplasty (BAV) for congenital aortic valve stenosis. We sought to use aortic valve morphologic features to discriminate between valves that respond favorably or unfavorably to BAV, using aortic insufficiency (AI) as the primary outcome. All patients who underwent BAV at 2 large-volume pediatric centers from 2007 to 2014 were reviewed. Morphologic features assessed on pre-BAV echo included valve pattern (unicuspid, functional bicuspid, and true bicuspid), leaflet fusion length, leaflet excursion angle, and aortic valve opening area and on post-BAV echo included leaflet versus commissural tear. Primary end point was increase in AI (AI+) of ≥2°. Eighty-nine patients (median age 0.2 years) were included in the study (39 unicuspid, 41 functional bicuspid, and 9 true bicuspid valves). Unicuspid valves had a lower opening area (p <0.01) and greater fusion length (p = 0.01) compared with functional and true bicuspid valves. Valve gradient pre-BAV and post-BAV were not different among valve patterns. Of the 16 patients (18%) with AI+, 14 had leaflet tears (odds ratio 13.9, 3.8 to 50). True bicuspid valves had the highest rate (33%) of AI+. On multivariate analysis, leaflet tears were associated with AI+, with larger opening area pre-BAV and lower fusion length pre-BAV. AI+ was associated with larger pre-BAV opening area. Gradient relief was associated with reduced angle of excursion. Valve morphology influences outcomes after BAV. Valves with lesser fusion and larger valve openings have higher rates of leaflet tears which in turn are associated with AI.
For the past 3 decades, balloon aortic valvuloplasty (BAV) has been commonly used to relieve stenosis in infants and children with congenital aortic valve stenosis (AS). Generally, the results are favorable after BAV, although many patients require reinterventions in the form of repeat BAV, surgical repair, or valve replacement. The development of aortic insufficiency (AI) after BAV has been noted to play an important role in the risk of valve reintervention and replacement. Children who develop significant AI post-BAV are at greater risk of valve repair and replacement. For this reason, there has been emerging interest in alternate approaches to congenital AS which may result in less severe AI. For example, some centers now suggest primary surgical valvotomy particularly in neonates, for whom, the risk of AI is greatest. However, it may be possible to discriminate, based on valve anatomy, those patients who are more likely to respond favorably to BAV. Previous studies have suggested that valve morphology pattern and leaflet thickness, for example, influence the immediate outcomes after BAV. In fact, valve morphology may even explain the timing and severity of presentation in children with congenital AS. We sought to determine which aortic valve morphologic features were associated with unfavorable tears, and in particular, which features were more likely to be associated with the development of AI. We hypothesized that greater preintervention anatomic characterization of the aortic valve would allow for enhanced patient selection which ultimately may result in improved outcomes after BAV.
Methods
All patients who underwent BAV from 2007 to 2014 at 2 large-volume pediatric centers and were aged ≤21 years at the time of the procedures were considered for inclusion in the study. Patients were included if high-quality pre-BAV and post-BAV echocardiograms were available within 30 days before and after the procedures. A high-quality echocardiogram was defined as a study in which precise valve morphology description and valve metric assessment were possible in the appropriate parasternal short-and long-axis views. Studies where these views were not available were excluded from review, and such patients, if there were no other echocardiograms available for review within the 30-day period, were excluded from the study. Patients who had pre-BAV or post-BAV echocardiograms that did not include parasternal short-axis imaging of the aortic valve en face, for example, were not included in this study. Patients who did not undergo an adequate echocardiogram within the 30-day pre-BAV or 30-day post-BAV echocardiogram were excluded. Patient characteristics, including gender, age, weight, and body surface area (BSA), were noted.
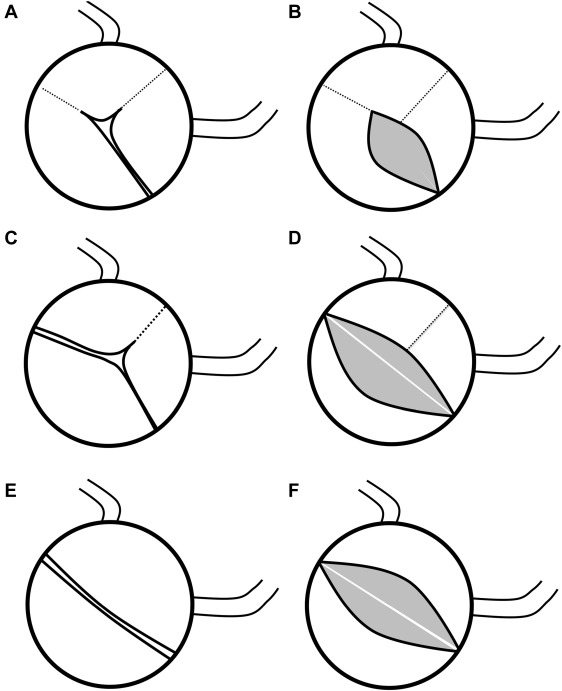
Aortic valves were comprehensively evaluated on pre-BAV and post-BAV echocardiograms for each patient. Each echocardiogram was independently assessed by 2 of 4 total reviewers (CJP, KG, SML, and RS). Regular imaging review sessions between the 2 participating centers occurred through video conferencing to ensure consistent valve morphologic description, measurement, and morphologic assignment. Reviewers were blinded to the clinical outcomes of the patients at the time of image review. Valve morphologic pattern ( Figure 1 ) was defined as 1 of 3 morphologic types: unicuspid when there was partial or complete fusion of 2 commissures, with 3 distinctly visible leaflets; functionally bicuspid when 3 distinct leaflets were noted but there was partial or complete fusion of only 1 commissure; true bicuspid when 2 distinct leaflets were noted with a single commissure and no appreciable raphe ( Figure 2 ). A number of valve characteristics were measured ( Figure 3 ) including: angle of excursion (angle) which was defined as the angle between the plane of valve hinge points and the tip of the anterior valve leaflet seen on parasternal long-axis view of the valve; valve leaflet asymmetry, defined as the ratio between largest leaflet surface area divided by smallest leaflet surface area; raphe (fusion) length, defined as the length of fusion from annulus edge, which was corrected for aortic valve annulus diameter; aortic valve opening area, which was defined as the cross-sectional area of the opening aortic valve at end systole (defined as end of the T wave) on parasternal short-axis view. Opening area was normalized to aortic valve annulus area, which was defined as the cross sectional area of the aortic valve using the annulus as the perimeter. Other data obtained from echocardiograms included the severity of AI on a 4-point scale (0 = none, 1 = mild, 2 = moderate and 3 = severe) according to published guidelines from the American Society of Echocardiography. Aortic valve annulus diameter, sinotubular junction diameter, ascending aortic diameter 1 cm above the sinotubular junction, pulmonary valve annulus diameter, left ventricular (LV) end-diastolic dimension, LV end-systolic dimension, and LV shortening fraction. These echocardiographic measurements were indexed to BSA where appropriate.
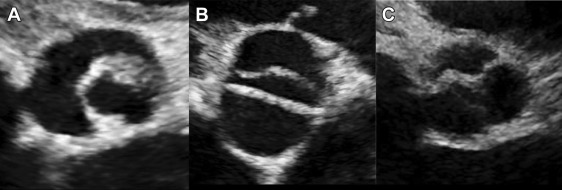
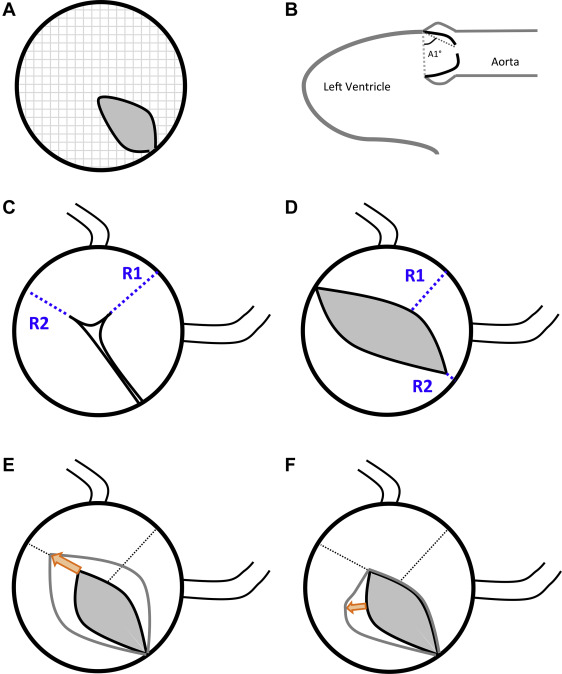
Post-BAV echocardiograms were reviewed by the same independent reviewers to assess for the presence, location, and degree of valve leaflet or commissural tear. Tears were categorized as favorable tears (commissural extensions) if there was extension of the commissure toward the aortic valve annulus and there was no new leaflet tear ( Figure 3 ). Unfavorable tears, also called leaflet tears, were defined as a new tear into an aortic valve leaflet that does not follow the path of a commissure ( Figure 3 ). No tear was noted if there was no extension of the fused commissure and no leaflet tear. The primary end point was AI+, which was defined as an increase in AI from pre-BAV to post-BAV of ≥2 points (this end point was based on the fact that AI+ is the outcome metric designated by the IMproving Pediatric and Adult Congenital Treatments registry of the American College of Cardiology/National Cardiovascular Data Registry). Significant AI was defined as greater than or equal to moderate AI. Among secondary end points was combined AS + AI, defined as a residual valve gradient >20 mm Hg in combination with significant AI.
Catheterization reports were reviewed for each patient. Aortic valve peak-to-peak gradient pre-BAV and post-BAV was noted. Patient characteristics at time of BAV were noted. Maximal balloon diameter was noted, and a balloon-to-aortic valve diameter ratio was recorded for each patient. Secondary end points included a resolution of stenosis, which was defined as a post-BAV gradient <30 mm Hg.
Data analyses were performed using SAS 9.3 (Cary, North Carolina), and statistical significance was evaluated at the 0.05 level. Descriptive statistics were calculated for all variables of interest by aortic valve morphologic pattern (unicuspid vs functional bicuspid and true bicuspid) using counts and frequencies, medians and ranges, or means and CIs. Interobserver agreement was ensured using interclass correlation with a value >0.7 ensuring strong agreement between observers. For leaflet tear versus commissural tear (dichotomous measure), a κ test valve >0.7 was considered good interobserver agreement. Results of interclass correlation and κ testing are presented in Table 1 . Comparisons between morphologic patterns were made using t tests for continuous variables and chi-square tests of independence in discrete cases. In situations of nonnormality, the t test was replaced by a nonparametric equivalent (Mann–Whitney U or Kolmogorov–Smirnov test); likewise, an exact form of the Pearson’s chi-square test was implemented when expected frequency counts were low (<5). Spearman-rank correlations, scatterplots, and line graphs were used to evaluate associations for continuous and categorical variables. Univariate and multivariable logistic regression models were used to identity significant risk factors associated with the 2 outcomes: AI and unfavorable leaflet tear. When sample sizes were small, standard estimates were replaced with those from exact logistic regression models. Potential risk factors were identified because of their historically known or demonstrated association with each outcome and included age, aortic valve morphologic pattern, pulmonary valve annulus diameter, raphe length/radius ratio, leaflet asymmetry, and the opening area/annulus area ratio, in others. Guided by the univariate results, forward selection techniques were used to build the multivariate models while checking for multicollinearity and potential interaction effects. Fit and predictive ability for each model was assessed through receiver operating characteristic curve statistics, and model-based risks of AI and unfavorable leaflet tear were plotted against increases in the opening area/annulus area ratio.
| Valve Characteristics – Continuous | ICC (95% CI) |
|---|---|
| AoV Opening Area | 0.876 (0.754 – 0.979) |
| AoV Annulus Area | 0.972 (0.949 – 0.976) |
| R-N Raphe Length | 0.837 (0.771 – 0.939) |
| R-L Raphe Length | 0.927 (0.886 – 0.980) |
| Anterior Angle of Excursion | 0.674 (0.362 – 0.719) |
| Valve Characteristics – Categorical ∗ | Kappa |
|---|---|
| Aortic Valve Type | 1.0 |
| Tear type (leaflet or commissural) | 0.94 |
∗ For categorical variables, Kendall coefficient of concordance was used.
Results
During the study period, 128 patients underwent BAV of which 89 patients (70%) had sufficient echocardiographic imaging. Thus, the study population consisted of 89 patients who met the inclusion criteria. For the aortic valve measurements described previously, there was either excellent or good agreement for each metric analyzed in the echocardiography reviewers ( Table 1 ). Unicuspid valves were noted in 39 patients (44%), whereas functionally bicuspid valves were noted in 41 patients (46%), and true bicuspid valves were noted in 9 patients (10%; Table 2 ). Although patients with unicuspid valves were younger, the pre-BAV valve gradient, degree of AI, and valve annulus size (corrected for BSA) were similar in the 3 groups ( Table 2 ). Patients with unicuspid valves had lower LV shortening fraction pre-BAV (p <0.01). The unicuspid cohort had greater overall valve fusion, noted by greater relative raphe (fusion) length (p = 0.010) and smaller aortic valve opening area (p <0.001) compared with both functionally bicuspid and true bicuspid valves. Other valve metrics, including angle of excursion, leaflet asymmetry, and aortic:pulmonary annulus diameter did not differ in morphologic types.
| Characteristic | Unicuspid N = 39 | Functionally Bicuspid N = 41 | True Bicuspid N=9 | P-Value |
|---|---|---|---|---|
| Age, N (%) | ||||
| < 1 Month | 23 (59%) | 11 (27%) | 0 (0%) | < 0.001 |
| < 1 Year | 35 (90%) | 20 (49%) | 3 (33%) | |
| ≥ 1 Year | 4 (10%) | 21 (51%) | 6 (67%) | |
| Age (years), Median (25 th – 75 th ) | 0.04 (0.01 – 0.20) | 3.58 (0.12 – 6.21) | 11.79 (2.1 – 16.21) | < 0.001 |
| Weight (kg), Median (25 th – 75 th ) | 3.60 (3.10 – 5.00) | 10.6 (3.3-44) | 32.1 (5.7-54) | < 0.001 |
| BSA (m 2 ), Median (25 th – 75 th ) | 0.22 (0.20 – 0.25) | 0.34 (0.21-1.4) | 1.1 (0.2-1.9) | < 0.001 |
| Annulus Diameter (cm)/BSA, Median (25 th – 75 th ) | 2.79 (2.52 – 3.15) | 2.51 (1.3-3.1) | 2.38 (1.2-2.7) | 0.002 |
| Raphe Length/Radius | 1.1 (0.87-1.3) | 0.87 (0.6-1.1) | 0.7 (0.3-1.0) | 0.010 |
| Opening Area/Annulus Area | 0.13 (0.07-0.2) | 0.23 (0.15 – 0.31) | 0.22 (0.2 – 0.4) | 0.001 |
| Angle Excursion (Anterior) | 53 (47 – 59) | 55 (46 – 63) | 53 (48 – 64) | 0.355 |
| LVSF Pre-BAV (%) | 30.1 (17 – 40) | 42.5 (35 – 47) | 38.0 (33 – 45) | < 0.001 |
| Mean Gradient Pre-BAV (mmHg) | 47 (32 – 56) | 46 (40 – 57) | 44 (40 – 59) | 0.306 |
| Mean Gradient Post-BAV (mmHg) | 24 (18 – 32) | 25 (19 – 32) | 15 (14 – 29) | 0.916 |
| Balloon-to-Annulus Ratio (25 th – 75 th ) | 1.06 (0.71-1.32) | 1.06 (0.96-1.14) | 1.07 (0.97-1.18) | 0.850 |
| Unfavorable Tear Present, N (%) | 6 (15.4%) | 6 (15%) | 4 (44%) | 0.144 |
| AI+, N (%) | 9 (23.1%) | 4 (10%) | 3 (33%) | 0.090 |
AI+ was encountered in 16 patients (18%). AI+ was seen in true bicuspid valves (3 of 9, 33%), in unicuspid valves (9 of 39, 23%), and in functionally bicuspid valves (4 of 41, 10%; p = 0.09). AI+ was highly associated with the presence of an unfavorable (leaflet) tear on post-BAV echocardiogram (odds ratio 13.9, CI 3.8 to 50) but was not associated with morphologic pattern, age at BAV, or other valve metrics ( Table 3 ). The rate of greater than or equal to moderate AI (n = 23) was equivalent in the morphologic types. The patient characteristics for true bicuspid valve and functional bicuspid valve morphologies were similar. Therefore, for purposes of multivariate analysis, the true bicuspid and functionally bicuspid valve cohorts were combined. Multivariate analysis indicated that AI+ was positively associated with valve opening area, and negatively associated with pulmonary valve annulus dimension, relative to aortic annulus dimension. Hence, the risk of AI+ increases as the pre-BAV valve opening area is greater ( Figure 4 ). In addition, the presence of an unfavorable (leaflet) tear post-BAV conferred a threefold risk of AI+. The risk of unfavorable tear, as seen in Figure 3 , likewise increases as the initial valve opening area is greater.
| Characteristic | OR (95% CI) | P-Value |
|---|---|---|
| Pulmonary Valve Annulus Diameter ∗ | 0.61 (0.39 – 0.95) | 0.030 |
| Opening Area/Annulus Area ∗ † | 1.55 (1.32 – 2.51) | 0.041 |
| Unfavorable Tear | 3.01 (1.41 – 4.61) | 0.01 |
| Morphology, Bicuspid | 0.58 (0.17 – 2.02) | 0.396 |
| BAR | 1.50 (0.88 – 1.99 ) | 0.172 |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


