Digoxin provides symptomatic relief in patients with systolic heart failure, yet it has potential proarrhythmic mechanisms and has not been formally studied in patients with cardiac resynchronization therapy-defibrillators (CRT-Ds). We evaluated the association between digoxin use and appropriate tachyarrhythmia therapy in patients with CRT-D with advanced heart failure, analyzing the incidence of appropriate device therapies and overall survival in 350 consecutive primary prevention recipients with CRT-D with baseline left ventricular ejection fraction (LVEF) ≤35%, non-right bundle-branch block native QRS complex ≥120 ms, New York Heart Association III to IV heart failure, and significant coronary artery disease. Digoxin was prescribed in 162 patients (46%) at discharge from CRT-D implant. Over 48 ± 32 months of follow-up, 59 patients (17%) received ≥1 appropriate shock. Digoxin therapy was associated with shorter time to first shock in intention-to-treat (corrected hazard ratio 2.18, 95% confidence interval 1.23 to 3.87, p = 0.007) and on-treatment analysis (corrected hazard ratio 2.27, 95% confidence interval 1.27 to 4.07, p = 0.006). Patients prescribed digoxin had a lower baseline LVEF, and digoxin therapy was associated with increased risk of shocks only in patients with LVEF <22% (median); there was no increased risk in patients with LVEF ≥22%. Overall survival and incidence of antitachycardia pacing were similar regardless of digoxin therapy. In conclusion, digoxin therapy is associated with increased likelihood of appropriate CRT-D shocks for rapid ventricular arrhythmias in primary prevention patients with coronary artery disease, and this risk appears to be most evident in patients with more severe baseline LV dysfunction. Digoxin use should be reexamined prospectively in patients with CRT-D.
Patients who receive cardiac resynchronization therapy-defibrillators (CRT-Ds) may be at particular risk of device shocks because progressive heart failure (HF) is associated with increasing ventricular arrhythmia burden. These patients with advanced, symptomatic HF may derive symptomatic benefit from digoxin. We queried an active database of patients with CRT-D at the University of Pittsburgh Medical Center to determine whether digoxin use is associated with an increased risk of appropriate ventricular tachyarrhythmia therapy in patients who received CRT-D for primary prevention against sudden cardiac death. Patients with significant coronary artery disease were examined exclusively because in our center’s CRT-D experience, patients with ischemic heart disease have a greater risk of appropriate device shocks, and myocardial ischemia potentiates digoxin’s potential for proarrhythmia.
Methods
We analyzed 350 consecutive patients who underwent CRT-D implantation between February 2000 and November 2012 from a prospectively maintained database at the University of Pittsburgh Medical Center. All patients were followed up in device clinic and met the following baseline criteria: (1) left ventricular ejection fraction (LVEF) ≤35%, (2) New York Heart Association class III to IV HF, (3) native QRS duration ≥120 ms with non-right bundle-branch block morphology, and (4) significant coronary artery disease. Significant coronary artery disease was defined as previous revascularization, ≥80% lesion in a major epicardial coronary artery, or a history of myocardial infarction with corroborating electrocardiogram, biomarker, or noninvasive imaging evidence. This definition is similar to that used in posthoc analyses of previous CRT-D studies. All patients received a defibrillator for primary prevention against sudden cardiac death, having no previous sustained, spontaneous ventricular arrhythmias. Sustained ventricular arrhythmias were defined as either ventricular tachycardia (VT) or ventricular fibrillation ≥30 seconds in duration or causing hemodynamic compromise requiring immediate therapy. The research described herein was approved by the Institutional Review Board of the University of Pittsburgh.
CRT-D devices were implanted by electrophysiologists using standard transvenous techniques. Right atrial pacing leads were actively fixed within the right atrial appendage or right atrial free wall. Defibrillator leads were placed within the apical right ventricular septum using active fixation leads. LV leads were inserted into a venous branch of the coronary sinus, with specific locations chosen for stability, acceptable capture thresholds, lack of phrenic nerve stimulation, and anatomic suitability. Bradycardia and tachycardia parameters were programed by the implanting physician. Antitachycardia pacing (ATP) in the ventricular fibrillation zone during charging was activated in all devices capable of this feature.
Patients were seen every 6 months in the clinic if not participating in home monitoring. Patients enroled in home monitoring were seen at least yearly, with device interrogations performed remotely every 3 months. Patients reporting device shocks, either performed a manual remote download, were seen within 48 hours in the clinic, or reported to a hospital emergency department. All electrograms were analyzed by an electrophysiologist at the time of presentation and reviewed during data acquisition. The median ventricular rate recorded by the device before delivery of any tachycardia therapy was noted. Device reprogramming, medication changes, ablation procedures, and/or hospital admission were at the discretion of the treating physician(s).
Patients referred for CRT-D were prescribed maximally tolerated doses of β-adrenergic antagonists and angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs). Digoxin, loop diuretics, and mineralocorticoid receptor antagonists were used at the discretion of treating physicians in a nonrandomized fashion. Medical therapy at the time of discharge from CRT-D implantation was used in the primary data analysis. In addition, an on-treatment analysis was performed using medical therapy at the time of the first appropriate CRT-D shock or, for patients with no appropriate shocks, at the time of most recent clinical follow-up. Follow-up medical therapy data were available in 326 patients (93%).
Categorical variables are listed as absolute numbers and percentages and were compared using the chi-square or Fisher’s exact test, as appropriate. Continuous variables are expressed as mean ± 1 SD and were compared using the t test for normally distributed variables and Wilcoxon Signed-Rank test for nonparametric distributions. Time-dependent outcomes were analyzed using Kaplan-Meier estimates and compared using the log-rank test. Multivariate analyses were carried out using baseline variables differing with a p value ≤0.1 and Cox regression for time-dependent outcomes. Ventricular tachyarrhythmias treated with unsuccessful ATP followed by a shock were categorized as a shock only. Patients were censored at the time of cardiac transplant or implantation of a ventricular assist device for survival analyses. p Values ≤0.05 were considered statistically significant. Statistical analyses utilized SPSS, version 21.0 (IBM Inc., Armonk, New York).
Results
We analyzed 350 patients whose baseline characteristics are listed in Table 1 . At the time of discharge from CRT-D implant, 162 patients (46%) were prescribed digoxin, and 42 patients (12%) were taking amiodarone or any class III antiarrhythmic drug for atrial arrhythmias. Previous revascularization (≥3 months) had been performed in 289 patients (83%), including bypass surgery in 201 patients. Of the 61 nonrevascularized patients, 45 had undergone diagnostic coronary angiography, including 21 patients with ≥1 chronically occluded vessel.
| Variable | Overall (n = 350) | Digoxin at Implant | p Value ∗ | |
|---|---|---|---|---|
| Yes (n = 162) | No (n = 188) | |||
| Men | 281 (80) | 124 (77) | 157 (84) | 0.10 |
| Age (yrs) | 70 ± 10 | 69 ± 10 | 71 ± 10 | 0.19 |
| NYHA class (mean) | 3.1 ± 0.3 | 3.1 ± 0.3 | 3.1 ± 0.3 | 0.86 |
| III | 325 (93) | 150 (93) | 175 (93) | 0.86 |
| IV | 25 (7) | 12 (7) | 13 (7) | 0.86 |
| Diabetes mellitus | 144 (41) | 68 (42) | 76 (41) | 0.80 |
| Glomerular filtration rate (ml/min) | 59 ± 22 | 58 ± 21 | 59 ± 24 | 0.77 |
| Paroxysmal atrial fibrillation | 100 (29) | 51 (32) | 49 (26) | 0.29 |
| Previous revascularization | 289 (83) | 134 (83) | 155 (82) | 0.40 |
| LVEF (%) | 22 ± 6 | 21 ± 6 | 23 ± 6 | 0.001 |
| QRS duration (ms) | 162 ± 25 | 165 ± 26 | 160 ± 24 | 0.06 |
| Left bundle-branch block | 258 (74) | 125 (77) | 133 (71) | 0.17 |
| Nonspecific intraventricular conduction delay | 92 (26) | 37 (23) | 55 (29) | 0.17 |
| β-adrenergic antagonist | 295 (84) | 136 (84) | 159 (85) | 0.87 |
| ACE-I or ARB | 286 (82) | 134 (83) | 152 (81) | 0.65 |
| Mineralocorticoid antagonist | 79 (23) | 48 (30) | 31 (17) | 0.003 |
| Statin | 239 (68) | 114 (70) | 125 (67) | 0.44 |
| Loop diuretic | 281 (80) | 143 (88) | 138 (73) | <0.001 |
| Daily furosemide dose (mg) † | 67 ± 69 | 76 ± 72 | 59 ± 66 | 0.02 |
| Antiarrhythmic agent | 42 (12) | 21 (13) | 21 (11) | 0.61 |
| Amiodarone | 35 (10) | 17 (11) | 18 (10) | 0.78 |
| Dofetilide | 3 (1) | 2 (1) | 1 (1) | 0.60 |
| Sotalol | 4 (1) | 2 (1) | 2 (1) | >0.99 |
| VF rate cutoff (beats/min) | 202 ± 15 | 202 ± 14 | 202 ± 14 | 0.88 |
| VT rate cutoff (beats/min) | 174 ± 12 | 175 ± 12 | 173 ± 12 | 0.54 |
∗ p Value reflects comparison of patients prescribed digoxin and those not prescribed digoxin at discharge from CRT-D implant.
† The equivalent furosemide dose was used for alternative loop diuretics.
Medical therapy for HF was relatively consistent between baseline and follow-up, in that a similar number of patients remained on digoxin, β-adrenergic antagonists, ACE-I or ARBs, and aldosterone antagonists at implant and follow-up ( Table 2 ). Digoxin and β-adrenergic antagonist use were most consistent, in terms of both absolute numbers of patients and individual use. ACE-I and ARB use declined slightly, and although the number of patients prescribed aldosterone antagonists remained stable, use of these agents demonstrated the most flux in terms of individual patients. The mean baseline furosemide (or its equivalent) dose was 67 ± 70 mg at baseline and increased to 71 ± 69 mg at follow-up (p = 0.08).
| Implant ∗ | Follow-Up | Persisted | Initiated | Discontinued | Remained Off | Status Unchanged (%) | |
|---|---|---|---|---|---|---|---|
| Digoxin | 146 | 148 | 129 | 19 | 17 | 163 | 83 |
| β-adrenergic antagonist | 277 | 286 | 262 | 24 | 15 | 25 | 82 |
| ACE-I or ARB | 269 | 235 | 215 | 20 | 54 | 37 | 72 |
| Mineralocorticoid antagonist | 73 | 68 | 41 | 27 | 32 | 226 | 76 |
∗ The number of patients is smaller than that in Table 1 because only patients with follow-up data (n = 326, 93%) are included.
There were few significant baseline differences between patients prescribed digoxin and those not taking digoxin at the time of CRT-D implant ( Table 1 ). Specifically, patients prescribed digoxin had a lower LVEF, were more frequently taking spironolactone or eplerenone, and were taking a higher daily furosemide dose. Patients prescribed digoxin tended to have a wider QRS duration, although this was not statistically significant. At follow-up, patients initially prescribed digoxin remained more likely to be using aldosterone antagonists (28% vs 16%, respectively, p = 0.007) and had become more likely to be prescribed an ACE-I or ARB (78% vs 67%, respectively, p = 0.04). The proportion of patients using β-adrenergic antagonists was similar at follow-up between patients initially on digoxin (88%) and off digoxin (87%, p = 0.8), but patients on digoxin were no longer using a higher daily dose of furosemide (75 ± 72 vs 68 ± 67 mg, respectively, p = 0.4).
Over 48 ± 32 months of follow-up, 59 patients (17%) experienced an appropriate device shock, of whom 37 patients (63%) were prescribed digoxin at the time of device implant (p = 0.006). At the time of first appropriate CRT-D shock, 39 patients were actually taking digoxin, representing 66% of those with an appropriate shock. Of these 39 patients, 36 were prescribed digoxin at the time of CRT implant, whereas digoxin had been initiated subsequently in 3 patients. Of the 37 patients with an appropriate shock who were prescribed digoxin at the time of CRT-D implant, 36 continued to take digoxin at the time of the shock, and 1 had discontinued the drug. Therefore, more patients with an appropriate shock had crossed over to digoxin therapy than had discontinued digoxin since device implant.
Using a time to first event analysis, patients prescribed digoxin were >2× more likely to receive an appropriate shock (hazard ratio [HR] 2.31, 95% confidence interval [CI] 1.36 to 3.92, p = 0.002; Figure 1 ). Correcting for LVEF, aldosterone antagonist use, QRS duration, gender, and baseline furosemide dose, this association persisted (HR 2.18, 95% CI 1.23 to 3.87, p = 0.007). On-treatment analysis also demonstrated that digoxin was associated with a >twofold shorter time to first appropriate shock (HR 2.42, 95% CI 1.41 to 4.15, p = 0.001), and this persisted after correcting for baseline LVEF, aldosterone antagonist and ACE-I or ARB use at follow-up, baseline QRS duration, and gender (HR 2.27, 95% CI 1.27 to 4.07, p = 0.006). Other than digoxin use, no variable in Table 1 was associated with time to first shock. Younger age, aldosterone use, baseline furosemide dose, and lower LVEF were each associated with trends toward shorter time to first shock (p = 0.06 to 0.10).

The mean ventricular rate at the time of device shock was 232 ± 40 beats/min (median 229). ATP was delivered immediately before the first appropriate CRT-D shock in 15 patients (25%). In only 3 patients did burst ATP accelerate the VT, necessitating a shock. In one other patient, an initial ATP burst failed to terminate the tachycardia but did not accelerate it, and only subsequent ramp ATP accelerated the tachycardia.
The daily furosemide dose at the time of first appropriate CRT-D shock (81 ± 69 mg [median 80]) was not significantly higher than the dose at implant (71 ± 65 mg [median 60]) in patients who did receive a shock (p = 0.15). Furthermore, the plasma potassium concentration was 4.2 ± 0.5 mM/L (median 4.2) at the time of first appropriate CRT-D shock. The potassium concentration (3.5 to 4.9 mM/L) was outside the normal range for our laboratory in only 4 patients (7%), among whom these values were 2.6, 3.0, 3.3, and 5.1 mM/L, respectively. The median daily digoxin dose at the time of first appropriate CRT-D shock was 0.125 mg, including 12 patients using 0.25 mg/d, 25 patients using 0.125 mg/d, and 2 patients taking 0.0625 mg/d.
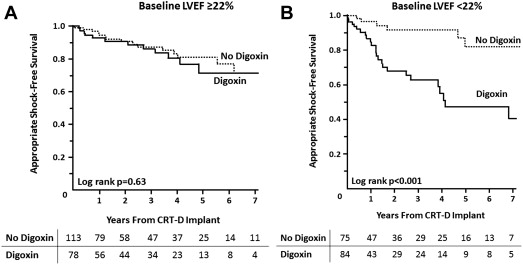
Among patients prescribed digoxin at device implant, the only baseline characteristic in Table 1 associated with shorter time to first appropriate shock was lower LVEF. To assess whether the association of digoxin with appropriate CRT-D shocks may have a differential relation based on baseline LVEF, the cohort was stratified according to the median (and mean) LVEF of 22%. There were 191 patients with LVEF ≥22% at baseline, of whom 78 patients (41%) were taking digoxin at device implant. In this group, 27 patients (14%) received an appropriate shock, of whom 12 patients (44%) were prescribed digoxin. This was not a statistically greater proportion than the 66 patients (40%) using digoxin of 164 patients without a shock (p = 0.7). There was no difference in time to first appropriate shock based on digoxin use in patients with baseline LVEF ≥22% ( Figure 2 ). In contrast, digoxin was associated with significantly shorter time to first appropriate shock in the 159 patients with LVEF <22% at baseline ( Figure 2 ). In this group, 32 patients (20%) received a shock, of whom 25 patients (78%) were prescribed digoxin, compared with digoxin use in 59 patients (47%) of 127 patients without a shock (p = 0.001).