Cardiac resynchronization therapy (CRT) induces left ventricular (LV) reverse remodeling by synchronizing LV mechanical activation. We evaluated changes in segmental LV activation after CRT and related them to CRT response. A total of 292 patients with heart failure (65 ± 10 years, 77% men) treated with CRT underwent baseline echocardiographic assessment of LV volumes and ejection fraction. Time-to-peak radial strain was measured for 6 midventricular LV segments with speckle-tracking strain imaging. Moreover, the time difference between the peak radial strain of the anteroseptal and the posterior segments was calculated to obtain LV dyssynchrony. After 6 months, LV volumes, segmental LV mechanical activation timings, and LV dyssynchrony were reassessed. Response to CRT was defined as ≥15% decrease in LV end-systolic volume at 6-month follow-up. Responders (n = 177) showed LV resynchronization 6 months after CRT (LV dyssynchrony from 200 ± 127 to 85 ± 86 ms; p <0.001) by earlier activation of the posterior segment (from 438 ± 141 to 394 ± 132 ms; p = 0.001) and delayed activation of the anteroseptal segment (from 295 ± 155 to 407 ± 138 ms; p <0.001). In contrast, nonresponders (n = 115) experienced an increase in LV dyssynchrony 6 months after CRT (from 106 ± 86 to 155 ± 112 ms; p = 0.001) with an earlier activation of posterior wall (from 391 ± 139 to 355 ± 136 ms; p = 0.039) that did not match the delayed anteroseptal activation (from 360 ± 148 to 415 ± 122 ms; p = 0.001). In conclusion, responders to CRT showed LV resynchronization through balanced lateral and anteroseptal activations. In nonresponders, LV dyssynchrony remains, by posterior wall preactivation and noncompensatory delayed septal wall activation.
Cardiac resynchronization therapy (CRT) has been shown to induce sustained left ventricular (LV) reverse remodeling in patients with severe heart failure, with significant improvements in LV systolic function and cardiac metabolism and decrease in mitral regurgitation. These beneficial effects have been principally attributed to the restoration of synchrony within the LV and further equilibration of the activation and contraction of the posterolateral and septal walls. However, up to 30% to 40% of patients do not experience significant mid-term LV reverse remodeling after CRT. The absence of adequate resynchronization or the induction of LV dyssynchrony after CRT has been one of the pathophysiological factors related to a lesser degree of response to the therapy. Consequently, whereas responders to CRT seem to show resynchronization of the sequence of activation within the LV, nonresponders may exhibit a distinct sequence of activation after CRT that hampers LV reverse remodeling. Accordingly, in the present evaluation, changes in timing of segmental LV activation after CRT were assessed with 2-dimensional (2D) speckle-tracking echocardiography and related to CRT response.
Methods
A total of 292 patients with heart failure treated with CRT and who completed a 6-month follow-up period were selected from an ongoing registry. Optimally treated patients with heart failure (New York Heart Association functional class III or IV) with an LV ejection fraction (LVEF) ≤35%, who showed a wide QRS complex (>120 ms), were selected for CRT. Patients with recent myocardial infarction (≤3 months), decompensated heart failure requiring continuous intravenous therapy, or patients in atrial fibrillation and high beat-to-beat variability that would preclude 2D speckle-tracking radial strain analysis were excluded. Heart failure etiology was considered ischemic if previous myocardial infarction or revascularization or significant angiographic coronary disease was documented. According to the institutional protocol, clinical evaluation was performed before CRT implantation and included New York Heart Association functional class, exercise capacity using the 6-minute walk test, and quality of life assessment using the Minnesota Living with Heart Failure Questionnaire. In addition, all patients underwent 2D echocardiography at baseline to evaluate LV volumes and LVEF. In addition, LV dyssynchrony, the sequence of activation, and site of latest activation were evaluated with 2D radial strain speckle-tracking. At 6-month follow-up the clinical status was reevaluated and echocardiography was repeated to evaluate LV volumes, LVEF, LV dyssynchrony, and sequence of LV mechanical activation. Response to CRT was defined as a ≥15% decrease in LV end-systolic volume 6 months after CRT implantation. The changes in sequence of LV mechanical activation after CRT were related to the echocardiographic response. All clinical and echocardiographic data were prospectively entered into the departmental Cardiology Information System (EPD-Vision; Leiden University Medical Center, Leiden, the Netherlands) and retrospectively analyzed.
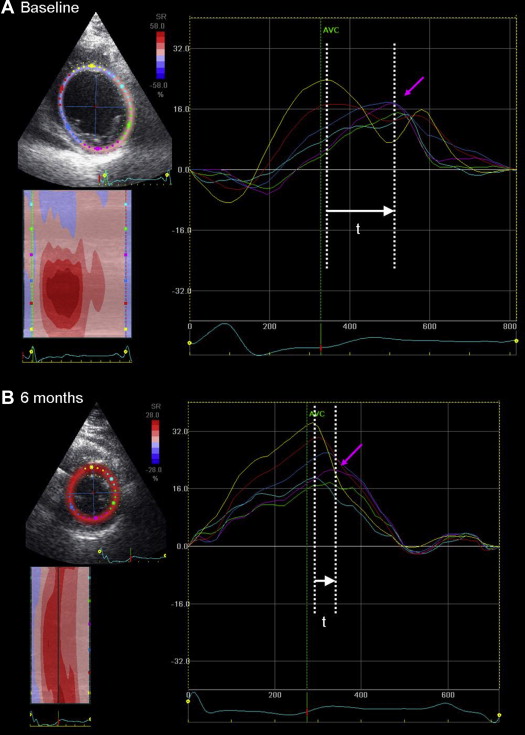
All echocardiographic data were obtained at rest in the left lateral decubitus position using a commercially available system (Vingmed System 7 and E9; GE-Vingmed Ultrasound AS, Horten, Norway) with a 3.5-MHz transducer. Apical 2- and 4-chamber views were used to calculate LV end-diastolic and end-systolic volumes and to derive LVEF according to Simpson’s method. LV volumes and ejection fraction were assessed before and 6 months after CRT. In addition, LV dyssynchrony, sequence of LV mechanical activation, and identification of the site of latest mechanical activation were assessed with 2D radial strain speckle-tracking. These parameters were evaluated using commercially available software (EchoPAC 111.0.00, GE-Vingmed, Horten, Norway) as previously described. The mid LV short-axis view was divided into 6 segments and the time-strain tracings are provided for each segment. The time difference between the peak radial strain of the anteroseptal and the posterior segments is calculated to define LV dyssynchrony ( Figure 1 ). An established cut-off value of ≥130 ms indicates significant LV dyssynchrony, as previously described. Furthermore, the time-to-peak radial strain of the 6 LV segments was recorded creating the sequence of mechanical activation of the LV. The latest activated segment was identified. A distinct sequence of activation was defined as <94 ms absolute timing of mechanical activation difference between a preactivated posterior wall and a delayed anteroseptal wall 6 months after CRT.
All CRT devices were implanted in the pectoral region and during implantation, pacing, sensing, and defibrillation thresholds were tested. CRT leads were implanted by way of the subclavian vein. First, a coronary sinus venogram was obtained. Thereafter, with the help of an 8-Fr guiding catheter, the LV lead was positioned (Easytrak, Guidant Corporation, St. Paul, Minnesota; Attain, Medtronic Inc., Minneapolis, Minnesota; or Corox, Biotronik, Berlin, Germany) by way of the coronary sinus, preferably in the posterolateral vein at the midventricular level. The right atrial and ventricular leads were positioned conventionally. A defibrillator was combined to CRT in all patients. Finally, all leads were connected to a dual-chamber biventricular CRT device (Contak Renewal, Guidant Corporation; Consulta, InSync III, or InSync Sentry, Medtronic Inc; or Lumax, Biotronik). The cardiologist performing the procedure was unaware of the site of latest mechanical activation identified on 2D speckle-tracking echocardiography.
According to protocol, all patients underwent a conventional chest x-ray before discharge to confirm the position of the LV lead. Using the lateral views, LV lead position was recorded as anterior, lateral, posterior, or inferior. Using the frontal views, the LV lead position was scored as basal, mid, or apical. Concordant LV lead position was defined by the present of an LV lead targeting the site of latest activation identified with speckle-tracking echocardiography.
Continuous variables are presented as mean ± SD, unless otherwise specified, and were compared between responders and nonresponders to CRT using the Student t test for unpaired data. In addition, the comparison between the timings of mechanical activation at baseline and at 6-month follow-up was performed using the Student t test for paired data. Categorical variables are presented as number and percentage and were compared with chi-square test. A multivariate logistic regression model was created introducing the significant univariate variables as covariates with the stepwise enter method to identify independent predictors of a distinct sequence of activation (<94 ms absolute timing of mechanical activation difference between a preactivated posterior wall and a delayed anteroseptal wall 6 months after CRT). For each variable, the odds ratio and the 95% confidence intervals were calculated. A p value <0.05 was considered statistically significant. Data were analyzed with SPSS 21.0 (IBM, Chicago, Illinois).
Results
The baseline characteristics of the population are listed in Table 1 . In most patients the LV lead was implanted at the midventricular level (91%). Concordant LV lead position was observed in 164 patients (60%). According to the prespecified definition of response (≥15% decrease in LV end-systolic volume at 6-month follow-up), 61% of patients were responders to CRT (n = 177) and 39% were nonresponders (n = 115). Comparisons between baseline characteristics in responders and nonresponders to CRT are outlined in Table 2 .
| Variable | n = 292 |
|---|---|
| Age (yrs) | 65 ± 10 |
| Men/Women | 227/65 |
| Coronary artery disease | 177 (61) |
| Sinus rhythm | 242 (83) |
| QRS duration (ms) | 157 ± 29 |
| LBBB | 213 (73) |
| QRS duration ≥130 ms | 237 (81) |
| NYHA class | |
| III | 273 (94) |
| IV | 19 (6) |
| 6MWT (m) | 311 ± 111 |
| QoL score | 36 ± 18 |
| LV end-diastolic volume (ml) | 218 ± 78 |
| LV end-systolic volume (ml) | 163 ± 68 |
| LVEF (%) | 26 ± 8 |
| LV dyssynchrony (ms) | 163 ± 121 |
| Site of latest activation (posterolateral) | 182 (63) |
| Medications | |
| β Blockers | 220 (75) |
| ACE inhibitors/ARB | 264 (90) |
| Diuretics/spironolactone | 242 (83) |
| Variable | Responders (n = 177) | Nonresponders (n = 115) | p Value |
|---|---|---|---|
| Age (yrs) | 64 ± 10 | 66 ± 10 | 0.047 |
| Men/Women | 135/42 | 92/23 | 0.274 |
| Coronary artery disease | 103 (56) | 74 (68) | 0.032 |
| QRS (ms) | 160 ± 29 | 150 ± 31 | 0.010 |
| LBBB | 138 (78) | 75 (65) | 0.017 |
| QRS ≥130 ms | 152 (86) | 85 (73) | 0.014 |
| NYHA | 3.1 ± 0.1 | 3.3 ± 0.2 | 0.840 |
| 6MWT (m) | 315 ± 104 | 305 ± 123 | 0.521 |
| QoL score | 34 ± 116 | 37 ± 19 | 0.784 |
| LV end-diastolic volume (ml) | 223 ± 82 | 208 ± 72 | 0.111 |
| LV end-systolic volume (ml) | 169 ± 73 | 152 ± 61 | 0.051 |
| LVEF (%) | 26 ± 7 | 27 ± 8 | 0.075 |
| LV dyssynchrony (ms) | 200 ± 127 | 106 ± 86 | <0.001 |
| Lateral or posterior site of latest mechanical activation | 145 (82) | 37 (32) | <0.001 |
| Patients with concordant LV lead position | 141 (79) | 27 (23) | <0.001 |
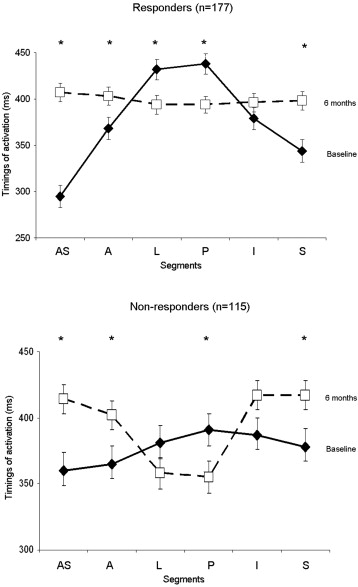
The sequence of activation in responders and nonresponders to CRT at baseline and after 6 months of CRT is illustrated in Figure 2 . Of interest, responders and nonresponders showed a comparable sequence of mechanical activation at baseline. The mechanical activation of the posterior wall was delayed compared with the anteroseptum in both responders and in nonresponders. However, different sequences of mechanical activation were observed after 6 months of CRT in responders and in nonresponders. In particular, responders experienced LV resynchronization at 6-month follow-up (LV dyssynchrony from 200 ± 127 to 85 ± 86 ms; p <0.001) by earlier activation of the posterior segment (438 ± 141 to 394 ± 132 ms; p = 0.001) and delayed activation of the anteroseptal segment (295 ± 155 to 407 ± 138 ms; p <0.001). In contrast, nonresponders showed worsening of LV dyssynchrony (from 106 ± 86 ms before CRT to 155 ± 112 ms 6 months after CRT; p = 0.001) with an earlier mechanical activation of posterior wall (391 ± 139 to 355 ± 136 ms; p = 0.039) that did not match the delayed anteroseptal wall (360 ± 148 to 415 ± 122 ms; p = 0.001).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


