In hypertension, angiotensin receptor blockers can augment regression of left ventricular (LV) hypertrophy. It is not known whether this also is the case after aortic valve replacement (AVR) for severe aortic stenosis (AS). To test the hypothesis that treatment with candesartan in addition to conventional treatment is able to augment LV and left atrial (LA) reverse remodeling in patients with AS undergoing AVR, we studied 114 patients scheduled for AVR. Patients were randomized to treatment with candesartan 32 mg 1 time/day or conventional therapy immediately after AVR. Patients were followed with echocardiographic evaluations 3, 6, and 12 months after surgery. Primary end point was change in LV mass index. At baseline and during follow-up no differences in systolic, diastolic, and pulse pressures were seen between groups. Baseline LV mass index was 134 ± 41 g/m 2 with no difference between groups. Mean decrease in LV mass index in the control group was 12 ± 28 g/m 2 compared to 30 ± 40 g/m 2 in the candesartan group (p = 0.015) during follow-up. After 12 months LV mass index was significantly lower in the candesartan group (103 ± 29 vs 119 ± 31 g/m 2 , p = 0.01). In addition, the candesartan group had greater improvement in longitudinal LV systolic function assessed by tissue Doppler S′ wave (0.6 ± 0.1-cm/s increase in control group vs 1.4 ± 0.1 cm/s in candesartan group, p = 0.01, p for trend = 0.02) and a decrease in LA volume (p for trend = 0.01). Treatment had no effect on diastolic E/e′ ratio or B-type natriuretic peptide. In conclusion, angiotensin receptor blockade with candesartan after AVR in patients with AS is associated with augmented reverse LV and LA remodeling compared to conventional management.
After aortic valve replacement (AVR) for degenerative aortic valve stenosis (AS), some degree of reverse remodeling with left ventricular (LV) hypertrophy regression occurs, but even several years after surgical intervention the left ventricle often remains abnormal. Although this may be related to patient prosthesis mismatch and poorly controlled arterial hypertension, the lack of regression is incompletely understood. Irrespective of cause, previous studies have demonstrated that incomplete regression of LV hypertrophy 12 to 18 months after AVR is an important predictor of adverse outcome. In patients with arterial hypertension and electrocardiographic evidence of LV hypertrophy, treatment with the angiotensin receptor blocker losartan has been demonstrated to induce regression of LV hypertrophy, which has been associated with improved survival. It is not known whether angiotensin receptor blockers immediately after AVR also can increase reverse remodeling. Thus, we hypothesized that in patients with symptomatic severe AS treatment with candesartan 12 months postoperatively would increase LV and left atrial (LA) reverse remodeling when initiated immediately after AVR.
Methods
The study was carried out as a single-center, consecutive; investigator-initiated study using a prospective randomized, blinded, end-point design. The study was registered with the National Board of Health and the Danish Data Protection Agency and was approved by the local ethical committee. All patients gave written informed consent. The trial has been registered at http://www.clinicaltrial.gov with identifier NCT00294775 .
Eligible were patients >18 years of age with symptomatic severe AS (estimated aortic valve area <1 cm 2 ) planned for AVR at Odense University Hospital (Odense, Denmark) from February 2006 to April 2008 and an adequate echocardiographic window. Patients with LV ejection fraction <40%, S-creatinine >220 μmol/L, previous aortic valve surgery, planned additional valve repair/replacement, infective endocarditis, severe aortic valve regurgitation, or ongoing treatment with an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker were excluded ( Figure 1 ). The need for AVR was decided by consensus between consulting cardiologists, cardiac surgeons, and anesthesiologists not taking part in the present study.
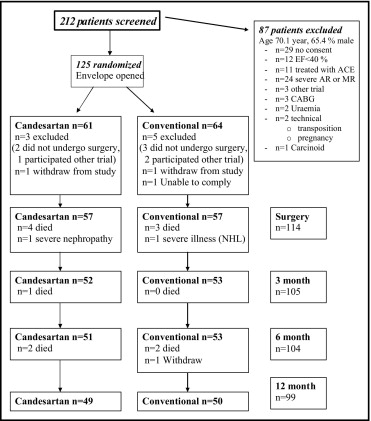
Patients were randomly assigned in 1:1 fashion to candesartan treatment or conventional treatment. Candesartan treatment was started the second day after surgery or on the day the patient was discharged from the cardiothoracic intensive care unit. Initial dose was candesartan 8 mg 1 time/day. If tolerated, the dose was increased to candesartan 16 mg after 2 days. After 4 weeks, the dose was again increased to target dose of 32 mg 1 time/day.
Clinical examination including a 6-minute walk test and electrocardiography was performed in all patients before surgery and repeated 3, 6, and 12 month after surgery. All patients were treated for arterial hypertension if brachial blood pressure at rest was >135/90 mm Hg at any controls. Blood pressure was measured 2 times from the right arm with a sphygmomanometer in a supine position after a 30-minute rest; average blood pressure was calculated. In the control arm calcium channel blockers and diuretics were preferred drugs to achieve blood pressure lowering.
All echocardiograms were obtained by the same experienced operator on a GE Vivid 5 ultrasound machine (GE Medical Systems, Horten, Norway) the day before surgery. Echocardiography was repeated 3, 6, and 12 month after surgery. Echocardiograms were stored digitally for later blinded analyses. Great care was taken to ensure random analyses of echocardiograms and complete blinding to randomization status and clinical variables in all cases.
Aortic valve area was estimated using quantitative Doppler using the continuity equation according to current guidelines. Peak flow velocity across the valve was determined in the window where the highest velocity could be recorded using continuous-wave Doppler aligned as parallel as possible with flow across the valve. Peak transvalvular gradient was estimated using the Bernoulli equation.
LV mass was estimated using the Devereux formula (0.8 × [1.04{LV internal diameter + posterior wall thickness + interventricular septal thickness} 3 – {LV internal diameter} 3 ] + 0.6 g). Diastolic LV wall thickness and dimensions were estimated from the average of 3 consecutive frozen 2-dimensional images obtained in the parasternal long axis. Relative wall thickness was calculated using the formula 2 × posterior wall thickness/LV end-diastolic diameter. LV hypertrophy was considered when LV mass index was >116 g/m 2 in men and >100 g/m 2 in women.
LV ejection fraction was estimated using the Simpson biplane method. Longitudinal LV systolic function was assessed using peak systolic mitral annular motion assessed with tissue Doppler imaging with the Doppler sample volume placed in the septal, lateral, anterior, inferior, and posterior mitral valve annuluses.
Mitral inflow was assessed in the apical 4-chamber view using pulse-wave Doppler with the sample volume paced at the tips of mitral leaflets during diastole. From the mitral inflow profile, E- and A-wave peak velocities and deceleration time were measured. Doppler tissue imaging of the mitral annulus was used in the aforementioned sampling sites to measure early diastolic e′ velocity from each site. A septal E/e′ ratio >15 or mean E/e′ ratio >13 was considered indicative of increased LV filling pressure. Diastolic filling pattern was categorized according to European Association of Echocardiography (EAE) guidelines. LA volume was assessed using the area–length method from the apical 4- and 2-chamber views. Measurements were obtained in end-systole from the frame preceding mitral valve opening; volume was indexed for body surface area. An LA indexed volume >32 ml/m 2 was considered abnormal.
Blood samples were collected after the subject had been resting for ≥30 minutes. Samples were collected in tubes containing ethylenediaminetetra-acetic acid. These were then centrifuged and plasma samples stored at −80°C for later analysis. N-terminal pro–brain natriuretic peptide (NT–pro-BNP) was determined using an ELECSYS pro-BNP immunoassay (Roche Diagnostics GmbH, Mannheim, Germany).
The prespecified primary end point for this study was change in LV mass index during follow-up. Secondary end points were changes in LA volume index, NT–pro-BNP, and E/e′ ratio.
An intent-to-treat analysis of all outcomes based on the original randomization was carried out. Data are presented as mean ± SD or number and percentage. Differences between groups were tested by Student’s t tests for unpaired data once normality was demonstrated and categorical variables using Fisher’s exact test. Due to a non-Gaussian distribution NT–pro-BNP is presented as median and interquartile range, and differences between groups were tested with a Wilcoxon rank-sum test. Serial changes in primary and secondary end points were tested using analysis of variance with repeated measures. Between-group differences were tested using the analysis of variance xtmixed test. Uni- and multivariable linear regression analyses were constructed to assess the relation between LV mass index at 12 months and randomization status after adjustment for possible confounders. A p value <0.05 was considered statistically significant. STATA/SE 9.0 (STATA Corp. LP, College Station, Texas) was used for statistical analysis.
Sample size was estimated based on a between-study SD of LV mass index of 20 g/m 2 and a clinically relevant difference between groups after 12 months of 10 g/m 2 . With an alpha of 0.05 and power of 90%, ≥90 patients were required.
Results
In the 114 randomized patients no differences in baseline characteristics, use of bioprosthesis, or concomitant need for coronary bypass surgery between the candesartan and control groups were seen ( Table 1 ). No difference in prevalence of arterial hypertension ( Table 1 ) and mean systolic or diastolic blood pressure was seen at baseline ( Table 2 ).
| Variable | Standard (n = 57) | Candesartan (n = 57) | p Value |
|---|---|---|---|
| Age (years) | 72.6 ± 10 | 72.3 ± 8 | 0.89 |
| Men | 37 (65%) | 34 (59%) | 0.70 |
| Hypertension | 23 (41%) | 24 (42%) | 1.00 |
| Diabetes mellitus | 7 (12%) | 11 (19%) | 0.44 |
| Atrial fibrillation | 9 (16%) | 8 (14%) | 1.00 |
| Coronary heart disease | 9 (16%) | 12 (21%) | 0.63 |
| Peripheral arterial disease | 4 (7%) | 7 (12%) | 0.53 |
| Stroke | 3 (6%) | 5 (9%) | 0.72 |
| Diuretic therapy | 19 (35%) | 23 (41%) | 0.56 |
| β-blocker therapy | 14 (25%) | 13 (23%) | 1.00 |
| Calcium channel blocker therapy | 12 (21%) | 11 (20%) | 1.00 |
| New York Heart Association classes III and IV | 15 (26%) | 16 (28%) | 1.00 |
| Chest pain | 28 (50%) | 29 (50%) | 1.00 |
| 6-Minute walk test (meters) | 350 ± 112 | 327 ± 136 | 0.40 |
| EuroSCORE | 5.7 ± 1.8 | 6.1 ± 2.0 | 0.25 |
| Logistic EuroSCORE | 5.1 ± 2.6 | 6.2 ± 5.2 | 0.17 |
| Mechanical prosthesis | 11 (20%) | 8 (14%) | 0.62 |
| Coronary artery bypass surgery | 16 (33%) | 20 (35%) | 0.55 |
| Valve size | 23.3 ± 3.7 | 23.6 ± 2.0 | 0.55 |
| Maze surgery | 7 (12%) | 3 (5%) | 0.32 |
| Variable | Conventional Treatment | Candesartan Treatment | ||||
|---|---|---|---|---|---|---|
| Preoperative (n = 57) | 6 Months (n = 46) | 12 Months (n = 50) | Preoperative (n = 57) | 6 Months (n = 47) | 12 Months (n = 49) | |
| Systolic blood pressure (mm Hg) | 145 ± 20 | 143 ± 17 | 144 ± 20 | 146 ± 22 | 142 ± 19 | 142 ± 25 ⁎ |
| Diastolic blood pressure (mm Hg) | 79 ± 12 | 79 ± 10 | 77 ± 10 | 79 ± 14 | 77 ± 11 | 75 ± 11 ⁎ |
| Pulse pressure (mm Hg) | 69 ± 13 | 70 ± 15 | 68 ± 10 | 70 ± 14 | 68 ± 13 | 70 ± 13 ⁎ |
| Diuretic therapy | 19 (33%) | 19 (41%) | 22 (44%) | 23 (40%) | 22 (47%) | 23 (47%) ⁎ |
| β-blocker therapy | 14 (25%) | 24 (52%) | 24 (48%) | 13 (23%) | 23 (49%) | 20 (41%) ⁎ |
| Calcium channel blocker therapy | 12 (21%) | 8 (17%) | 13 (26%) | 11 (19%) | 6 (13%) | 7 (14%) ⁎ |
In the 2 groups a significant decrease in LV mass index was seen during follow-up ( Figure 2 ). Mean decrease in LV mass index in the control group was 12 ± 28 compared to 30 ± 40 g/m 2 in the candesartan group (p = 0.015). Thus, at 12 months LV mass index was significantly lower in the candesartan group (103 ± 29 vs 119 ± 31 g/m 2 , p = 0.01; Table 3 ). Univariable predictors of change in LV mass from baseline to 12 months are listed in Table 4 . In multivariable regression analysis, baseline LV mass index, known history of hypertension, and treatment with candesartan remained significant predictors of change in LV mass index at 12 months ( Table 4 ). In the candesartan group, a statistically significant decrease of relative wall thickness was seen (−0.08 ± 0.02, p = 0.002), whereas there was a trend toward a decrease in the control group (−0.05 ± 0.02, p = 0.07). Thus, at 12 months relative wall thickness was significantly decreased in the candesartan group (0.52 ± 0.11 vs 0.57 ± 0.12, p = 0.03).
| Variable | Conventional Treatment | Candesartan Treatment | ||||
|---|---|---|---|---|---|---|
| Preoperative (n = 57) | 6 Months (n = 46) | 12 Months (n = 50) | Preoperative (n = 57) | 6 Months (n = 47) | 12 Months (n = 49) | |
| Left ventricular mass index (g/m 2 ) | 130 ± 32 | 125 ± 32 | 119 ± 31 | 137 ± 48 | 112 ± 42 ⁎ | 103 ± 29 ⁎ † |
| Relative wall thickness | 0.61 ± 0.13 | 0.57 ± 0.10 | 0.57 ± 0.12 | 0.60 ± 0.14 | 0.55 ± 0.13 | 0.52 ± 0.11 ⁎ † |
| Left ventricular hypertrophy | 37 (65%) | 31 (67%) | 28 (56%) | 36 (63%) | 20 (43%) ⁎ † | 18 (37%) ⁎ ‡ |
| Diastolic interventricular septal thickness (cm) | 1.28 ± 0.21 | 1.26 ± 0.21 | 1.24 ± 0.24 | 1.34 ± 0.23 | 1.19 ± 0.24 ⁎ | 1.13 ± 0.22 ⁎ † |
| Diastolic left ventricular posterior wall (cm) | 1.34 ± 0.24 | 1.27 ± 0.17 | 1.24 ± 0.20 ⁎ | 1.31 ± 0.22 | 1.21 ± 0.22 ⁎ | 1.13 ± 0.17 ⁎ † |
| Left ventricular end-diastolic diameter (cm) | 4.46 ± 0.58 | 4.49 ± 0.54 | 4.45 ± 0.61 | 4.51 ± 0.67 | 4.44 ± 0.61 | 4.46 ± 0.61 |
| Aortic valve area (cm 2 ) | 0.82 ± 0.27 | 1.61 ± 0.45 ⁎ | 1.66 ± 0.4 ⁎ | 0.81 ± 0.29 | 1.73 ± 0.45 ⁎ | 1.63 ± 0.40 ⁎ |
| Effective orifice area/body surface area (cm 2 /m 2 ) | 0.48 ± 0.18 | 0.91 ± 0.25 ⁎ | 0.97 ± 0.30 ⁎ | 0.46 ± 0.19 | 0.95 ± 0.27 ⁎ | 0.91 ± 0.24 ⁎ |
| Peak aortic valve velocity (m/s) | 3.9 ± 0.7 | 2.0 ± 0.4 ⁎ | 2.1 ± 0.4 ⁎ | 3.9 ± 0.9 | 2.0 ± 0.4 ⁎ | 2.1 ± 0.4 ⁎ |
| End-diastolic volume (ml) | 107 ± 31 | 102 ± 23 | 97 ± 26 | 113 ± 37 | 107 ± 31 | 104 ± 33 |
| Ejection fraction (%) | 54 ± 8 | 52 ± 8 | 53 ± 8 | 54 ± 7 | 55 ± 8 | 53 ± 8 |
| Mean S′ wave (cm/s) | 6.2 ± 1.2 | 6.9 ± 1.2 | 7.0 ± 1.2 ⁎ | 6.0 ± 1.5 | 7.1 ± 1.3 ⁎ | 7.5 ± 1.4 ⁎ |
| E wave (m/s) | 0.79 ± 0.25 | 0.88 ± 0.30 | 0.85 ± 0.27 | 0.82 ± 0.21 | 0.88 ± 0.25 | 0.92 ± 0.29 |
| A wave (m/s) | 0.98 ± 0.25 | 0.90 ± 0.25 | 0.92 ± 0.26 | 0.95 ± 0.28 | 0.93 ± 0.26 | 0.93 ± 0.22 |
| E-wave deceleration time (ms) | 207 ± 63 | 202 ± 57 | 199 ± 59 | 191 ± 55 | 200 ± 53 | 203 ± 51 |
| Lateral E′ wave (cm/s) | 7.4 ± 2.3 | 9.1 ± 2.6 ⁎ | 8.7 ± 1.9 ⁎ | 7.2 ± 2.7 | 9.5 ± 2.9 ⁎ | 9.1 ± 2.3 ⁎ |
| Septal E′ wave (cm/s) | 5.6 ± 1.7 | 6.2 ± 2.4 | 6.0 ± 1.6 | 5.7 ± 1.4 | 6.1 ± 1.6 | 6.2 ± 1.7 |
| Mean E′ wave (6 segments) | 6.3 ± 1.7 | 7.4 ± 2.1 ⁎ | 7.2 ± 1.5 | 6.3 ± 1.7 | 7.2 ± 1.3 ⁎ | 7.5 ± 1.7 ⁎ |
| E/E′ lateral | 11.5 ± 4.8 | 10.2 ± 4.1 | 10.2 ± 3.8 | 12.9 ± 5.7 | 10.1 ± 4.6 ⁎ | 11.0 ± 5.6 |
| E/E′ septal | 14.8 ± 5.2 | 15.8 ± 7.8 | 15.4 ± 7.3 | 15.3 ± 5.5 | 15.3 ± 6.0 | 16.1 ± 7.4 |
| Left atrial volume (ml) | 87 ± 34 | 90 ± 33 | 89 ± 34 | 87 ± 32 | 85 ± 29 | 80 ± 25 ⁎ |
| Left atrial volume index (ml/m 2 ) | 50 ± 18 | 51 ± 19 | 49 ± 18 | 50 ± 19 | 47 ± 17 ⁎ | 44 ± 15 ⁎ |
| Diastolic function | ||||||
| Grade 0 | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grade 1 | 22 (39) | 13 (32) | 14 (32) | 16 (30) | 14 (31) | 17 (40) |
| Grade 2 | 22 (39) | 19 (41) | 17 (39) | 26 (49) | 22 (49) | 17 (40) |
| Grade 3 or 4 | 11 (20) | 9 (22) | 13 (30) | 11 (21) | 9 (20) | 9 (21) |
| Pro–brain natruretic peptide, median (interquartile range) | 440 (194–1,340) | 340 (149–662) | 295 (148–515) | 425 (204–1,120) | 374 (160–956) | 379 (200–858) |
| Mild/moderate mitral regurgitation | 14 (25%) | 13 (28%) | 10 (20%) | 12 (21%) | 8 (17%) | 11 (22%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


