Before the introduction of modern imaging methods such as magnetic resonance imaging (MRI) and computed tomography (CT), electroencephalography (EEG) was routinely used to identify and localize intracranial pathology. Over the ensuing decades, electroencephalographers refined these findings and became somewhat adept at using them to localize superficial cerebral lesions. However, for deep lesions, particularly those in the posterior fossa, EEG can be limited, as the findings are typically nonspecific and diffuse.
In recent years, CT and MRI have replaced EEGs as diagnostic tests of choice for detecting and localizing cerebral lesions. Both are superior to EEG for these indications and provide useful information about etiology. EEG, however, is complementary and has usefulness in these settings. EEG provides physiologic function of the brain, information that is not readily available from imaging. Such information can be important in estimating the extent of a functional impairment, recognizing a coexistent metabolic or toxic encephalopathy, and indicating a lesion’s epileptic potential. Occasionally, as in acute ischemia, EEG changes may proceed or outlast imaging abnormalities. Combining imaging studies and EEG, therefore, will often give a more complete neurophysiological picture than will either study alone. In this chapter, we discuss the EEG associated with focal encephalopathies. Etiologies commonly associated with focal encephalopathies include cerebrovascular diseases, neoplasms, abscess, and other focal infections. Epileptiform and nonepileptiform abnormalities as it relates to these various lesions will be discussed. It is also important to understand that many neurological conditions are often accompanied by several EEG findings (Table 8.1). No one finding is diagnostic of any given condition.
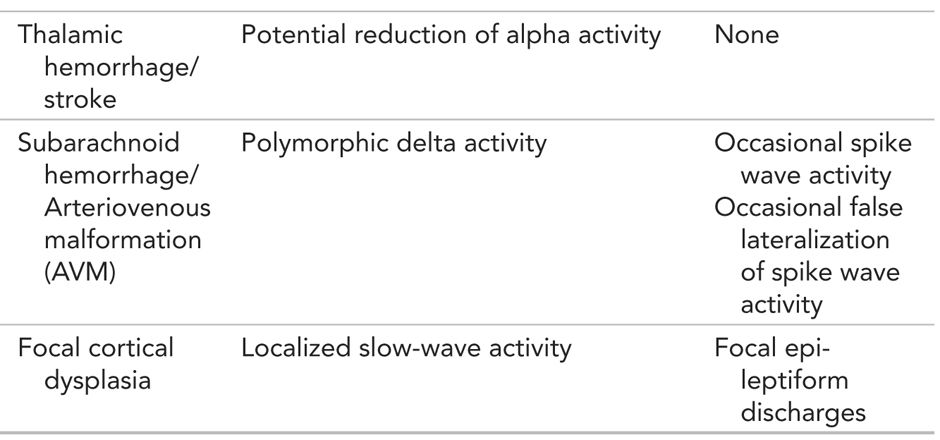
Summary: EEG Findings in Focal Encephalopathies

NONEPILEPTIFORM ABNORMALITIES
Changes in Background Rhythms
Changes in the EEG produced by focal brain lesions may be categorized as either epileptiform or nonepileptiform. Epileptiform abnormalities include spikes, sharp waves, spike-and-wave or sharp- and slow-wave discharges, and periodic discharges. Nonepileptiform abnormalities are of several types. First, localized slow activity is the most prevalent abnormality associated with focal brain lesions; the slowing is further classified as arrhythmic or rhythmic frontal intermittent delta activity. There can also be alterations in normally present cerebral rhythms such as frontal beta activity or the alpha rhythm. Background activity can be attenuated locally. Finally, widespread or diffuse abnormalities that occur with focal lesions, especially those located in the subfrontal cortex or thalamus where those that produce hydrocephalus could lead to nonspecific generalized changes.
Persistent asymmetry of beta activity indicates focal cortical dysfunction, which is often due to a cerebral lesion. The most common cause of focally enhanced beta activity is the breach rhythm. This is usually the consequence of intracranial surgeries requiring a craniotomy or burr hole (Figs. 8.1 and 8.2). Less often, the breach rhythm results from a skull fracture. Skull defects enhance the voltage of frontal fast rhythms as much as threefold. This is most evident at midcentral and temporal electrode sites because of their proximity to underlying mu rhythms or temporal rhythmic activity in the alpha frequency range. Although the enhanced beta activity is maximal nearest a burr hole or craniotomy margin, there is typically a broad voltage distribution. Focally enhanced beta activity is rare in other circumstances. It has been described with brain abscess, stroke, arteriovenous malformation (AVM), tumors, and focal cortical dysplasia. Localized attenuation of beta activity is much more common in these conditions than enhancement.
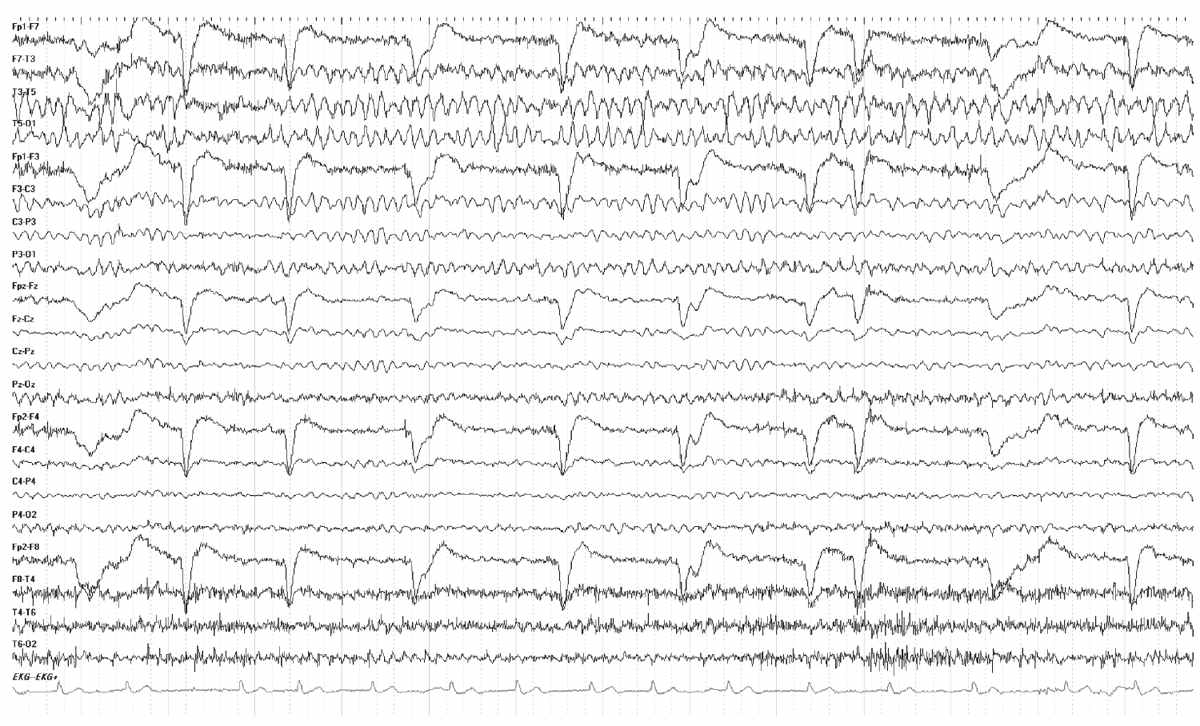
Figure 8.1: Breach Rhythm. EEG demonstrating a characteristic breach rhythm over the left hemisphere. Note the increased beta activity over the left hemisphere and higher-amplitude background rhythms.
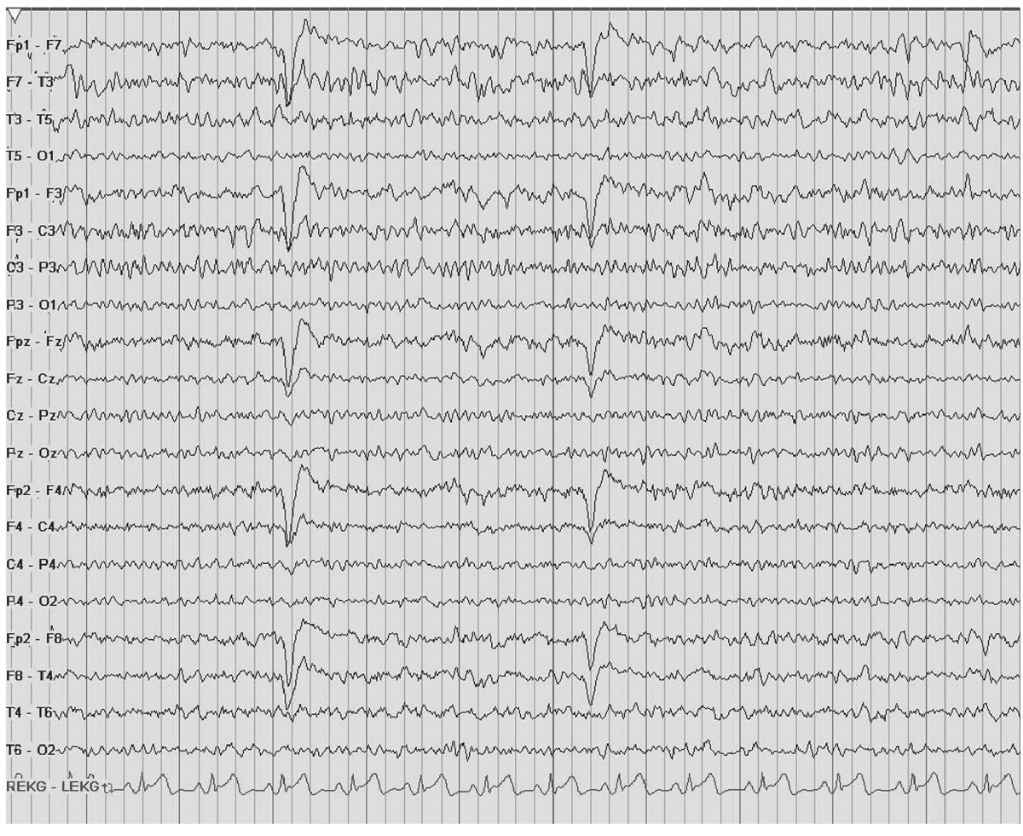
Figure 8.2: The EEG shows a typical Breach rhythm involving the left hemisphere. Note the increased amplitude over the left hemisphere along with increased beta rhythm. The patient has a history of a left temporal lobectomy.
Asymmetrical beta activity should be considered abnormal if there is persistent voltage difference of 35% or more between homologous areas of the two sides. Frontal beta activity tends to occur out of phase on the left and right sides, and a voltage asymmetry can therefore be enhanced using bipolar montages that connect homologous areas. Focal attenuation of beta activity is a reliable sign of cortical abnormality.
Focal attenuation of beta activity is seen in a number of conditions, including brain abscess, stroke, AVMs, and brain tumors. Green and Wilson [1] concluded that beta activity seems to be diminished with vascular lesions and enhanced by tumors. Barbiturates have also been used to bring out a beta asymmetry, because focal structural lesions often diminish the usual enhancing effect of such drugs. Beta activity is also attenuated by subdural, epidural, or subgaleal fluid collections. These selectively suppress higher-frequency activity, much like a fast-frequency filter. Focal slowing usually accompanies parenchymal lesions, but not fluid collections, a point that is sometimes helpful for interpretation. However, when extra-axial blood or cerebral spinal fluid results from a head injury, the underlying cortex is often injured as well, invalidating this distinction.
Focal cerebral lesions can alter the alpha rhythm even when they do not directly involve the occipital lobes and adjacent brain regions. Changes that can result from focal lesions include (a) unilateral slowing of frequency (1 Hz or more difference between the sides), (b) loss of reactivity, (c) loss of modulation, and (d) voltage attenuation. Unilateral attenuation or absence of alpha rhythm usually occurs with lesions of the occipital cortex and anterior ventral thalamus. Unilateral failure of the alpha rhythm to attenuation with eye opening (Bancaud phenomenon) occurs with posterior subcortical lesions (Fig. 8.3). Voltage asymmetry, in itself, is only rarely an indication of focal abnormality. In contrast, symmetries of frequency and reactivity are abnormal and reliably indicate the side of the lesion. Skull defects result in higher-voltage alpha rhythm, a difference that can be two to three times that of the normal side. Rarely, the alpha rhythm is higher on the side of the brain tumor, but in such cases, the alpha rhythm is also usually less reactive and poorly modulated.
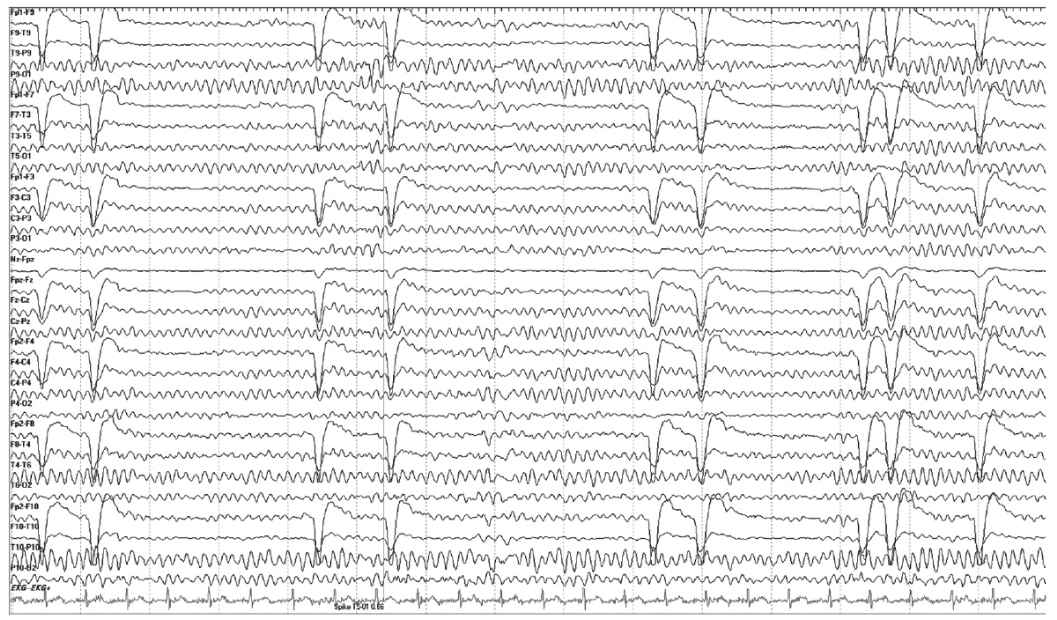
Figure 8.3: Bancaud phenomenon. In this EEG, we see a persistence of the alpha rhythm despite eye closure. The eye movement artifact confirms the phenomenon.
Asymmetries and the response to intermittent photic stimulation may result from lesions on the side of lower voltage, but responses can also be consistently lateralized in some normal individuals. Therefore, voltage asymmetries in photic driving should not be interpreted as abnormal in the absence of corroborative findings such as focal slowing or localized attenuation of background rhythms on the same side. Rarely, epileptogenic lesions result in responses to photic stimulation that are of higher voltage on the side of the lesion.
Normal sleep patterns, especially sleep spindles and vertex waves, can be affected by cerebral lesions. In sleep stage N2, as well as established persistent asymmetry of spindles indicate an abnormality on the side of lower voltage. Lesions of the parietal lobe or thalamus can attenuate sleep spindles. Thalamic lesions can also result in interhemispheric asynchrony of spindles, affect regulation spindle frequency, or rarely lead to the appearance of spindle-like rhythms during the waking state. Skull defects enhance the scalp-recorded voltage of sleep spindles and vertex waves.
Focal Slow-Wave Activity
Slow-wave activity is classified as arrhythmic or rhythmic, intermittent or continuous, focal or generalized. Focal polymorphic delta activity is slow-frequency activity, less than 4 Hz, that lacks sustained rhythmicity, characterized by constantly changing morphology, frequency, and voltage. Continuous focal polymorphic delta activity is highly correlated with a localized structural lesion such as tumor, stroke, abscess, intraparenchymal hematoma or contusion. Such delta activity usually persists during changes in physiologic states. The clinical correlation of intermittent polymorphic slow-wave activity is less well-defined as are those of delta waves, which attenuate with eye opening or disappear with sleep. Voltage of focal polymorphic delta activity is usually higher than ongoing background activity, but it can vary, depending, in large part, on the proximity of the lesion to the cortical surface and recording electrodes. Typical voltage of focal polymorphic delta activity is 100 to 150 μV in adults and up to 500 μV in children.
Focal polymorphic delta activity is often but not always maximal over the lesion. This activity implies that a sufficient destruction of the cortex in adjacent white matter (WM) has occurred. However, the voltage of the delta activity is actually reduced over the area of maximal cerebral involvement by the lesion. It is particularly important to pay attention to areas of relatively inactivity, flat or smooth polymorphic delta activity in which the voltage of both slow waves and faster frequencies are depressed. In these cases, the voltage will be higher in the areas bordering the lesion. The localizing value of focal polymorphic delta activity is greatest when it is topographically discrete and associated with depression of superimposed faster background frequencies. Superficial lesions tend to produce more restricted EEG changes, whereas deep lesions can result in hemispheric or even bilateral delta activity. Focal delta activity associated with lesions of the frontal lobe typically spreads to homologous contralateral areas where it is typically of lower voltage and has a smaller field than slowing ipsilateral to the lesion. Lesions involving the posterior, frontal, and parietal lobes often produce delta activity that is falsely localized to the temporal areas.
Bilateral synchronous delta or theta activity with an anterior predominance is often known as frontal intermittent rhythmic delta activity (FIRDA). First described by Cobb (2), this activity need not necessarily be frontal nor does it have to possess exclusively delta frequency activity; theta activity can also occur. IRDA (intermittent rhythmic delta activity) may be diffuse or lateralized with a sudden onset standing out from background EEG activity. In adults, the classic presentation is bifrontal where the acronym FIRDA applies, whereas in children, occipital predominance is noted and the acronym is OIRDA (occipital intermittent rhythmic delta activity). When IRDA is predominant in the temporal lobe and coupled with spike waves, the rhythm is known as temporal intermittent rhythmic delta activity (TIRDA).
FIRDA must be differentiated from vertical eye blinks; simultaneous recording of infraorbital and frontal scalp electrodes can easily distinguish between the two patterns. FIRDA is in phase with recordings taken above and below the eye (Fig. 8.4). Other benign rhythms that need to be distinguished from FIRDA include normal hyperventilation (HV) response, and hypnagogic hypersynchrony.
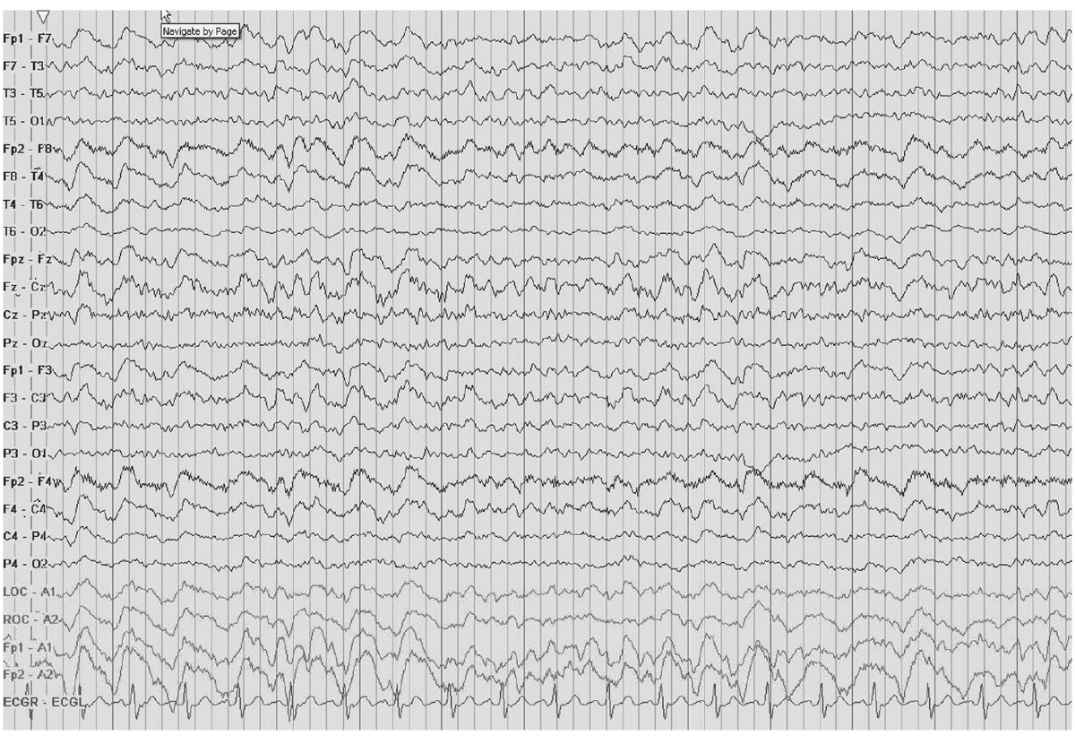
Figure 8.4: FIRDA detected with the use of eye leads. This EEG shows FIRDA and how the use of eye leads can help to distinguish between FIRDA versus eye movement artifact. Note how the delta activity is in phase with the extra eye lead channels at the bottom of the montage.
IRDA is an abnormal yet nonspecific finding in adults. TIRDA is the only pattern correlated to epilepsy (Fig. 8.5). Anatomically, IRDA may arise from structural brain lesions, yet is more commonly associated with diffuse encephalopathies. When associated with brain lesions, those abnormalities are anterior and hemispheric. Deep midline lesions, subcortical abnormalities, and increased intracranial pressure may also cause FIRDA (Figs. 8.6 and 8.7).
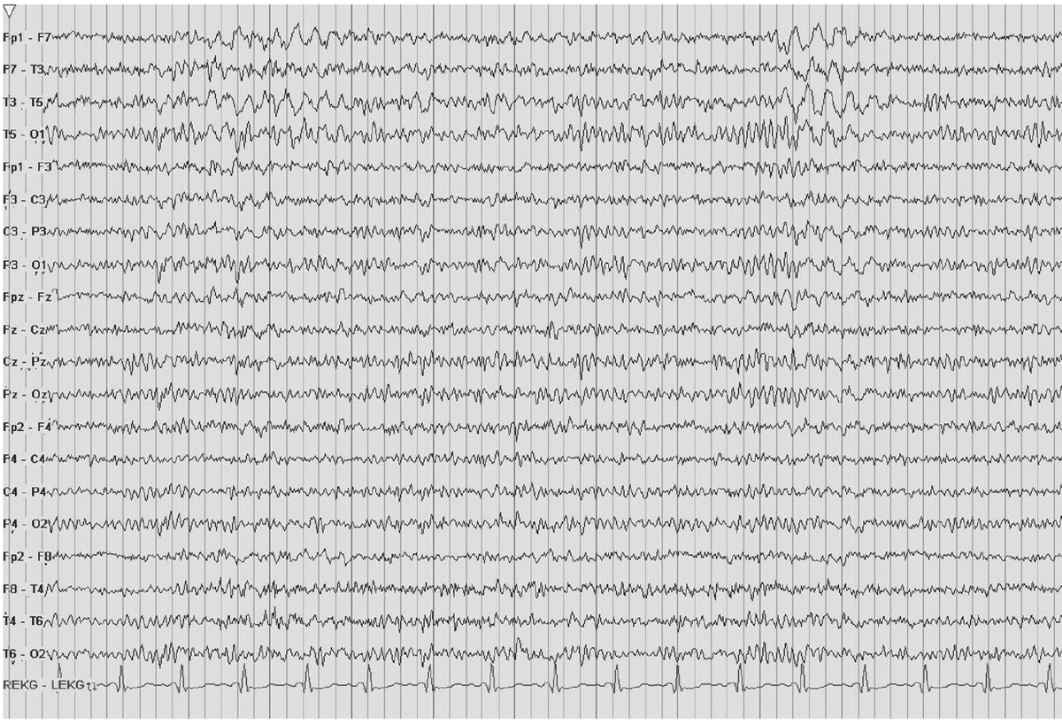
Figure 8.5: TIRDA. The EEG displays TIRDA. As compared to FIRDA or OIRDA, TIRDA is highly correlated to epilepsy.
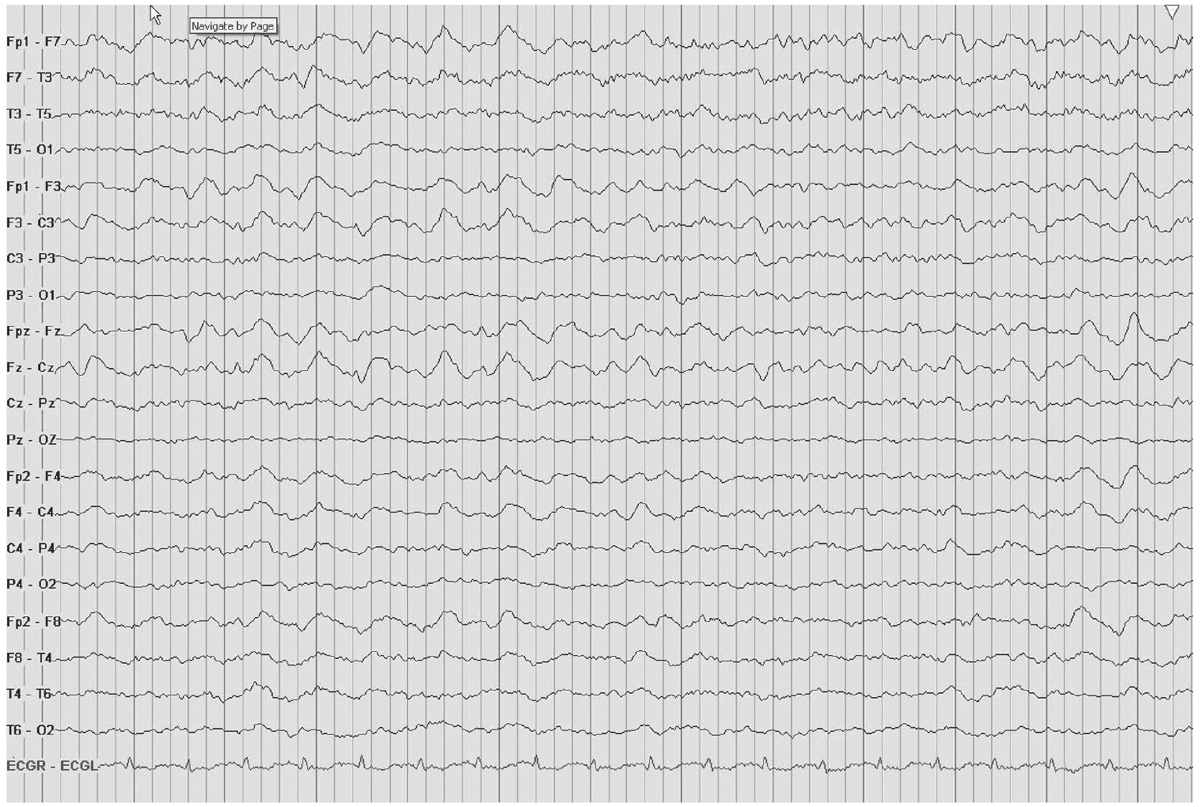
Figure 8.6: FIRDA. The EEGs here and in Fig. 8.7 show FIRDA from different causes. In this figure, FIRDA is secondary to a metabolic derangement in a patient with a urinary tract infection and confusion.
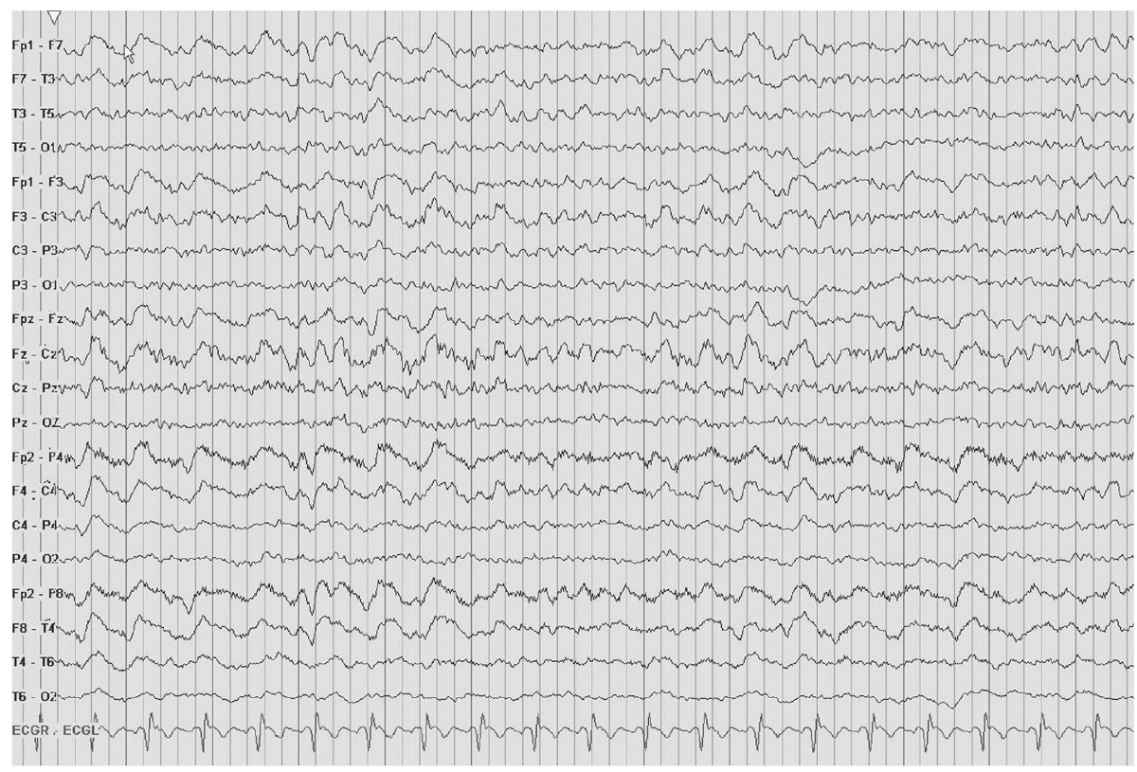
Figure 8.7: FIRDA. The patient has a history of nonspecific cognitive decline and multiple small WM changes on imaging.
Clinical and experimental data indicate that polymorphic delta activity usually results from lesions affecting the cerebral WM. Involvement of the superficial cortex is not essential; indeed, lesions restricted to the cortical mantle do not generally produce significant focal delta activity. Cerebral edema by itself does not make a substantial contribution to the production of delta waves. Brain tumors, abscesses, and areas of infarctions are electrically silent. The EEG changes produced by these lesions probably reflect alterations in cortical function caused (a) directly by anatomic disruption of neurons in their local networks or locally impaired blood flow, cellular metabolism in the micro environment; and (b) indirectly by modification of input to cortical neurons (3). EEG is generally not useful in distinguishing among different types of brain lesions. This is largely because there are only a limited number of ways the brain can react to injury; therefore, few electroencephalographic changes are seen with a wide variety of conditions affecting the central nervous system (CNS).
Although experimental, clinical, and brain imaging studies have demonstrated the strong correlation between localized anatomical pathology and focal polymorphic delta activity, they also illustrate that delta activity can occur in the absence of a demonstrable lesion. This is most likely to occur when the polymorphic slowing is intermittent and contains substantial amounts of intermixed theta activity. In these cases, the underlying cerebral dysfunction is often reversible. Examples include migraine, trauma, encephalitis, and postictal dysfunction. Focal arrhythmic theta activity can be seen early in the course of benign or well-differentiated brain tumors and during resolution of acute lesions caused by stroke or head trauma.
EPILEPTIFORM ABNORMALITIES
Seizures are a common final pathway for clinical presentation of several types of focal pathologies, so it should not be surprising that interictal epileptiform discharges are frequently present in the EEGs of such patients. Epileptiform activity is common in the EEGs of patients with well-differentiated glioma, traumatic injury, brain abscess, mesiotemporal sclerosis, and cortical dysplasia. In patients with benign or very slowly progressive tumors, focal epileptiform discharges sometimes antedate the appearance of focal slow-wave activity by months or years. In general, however, patients with brain tumors usually have other EEG abnormalities such as focal slowing, voltage attenuation, in addition to spikes or sharp waves. Epileptiform discharges are less common with acute cerebral infarction or hemorrhage. Although these typically occur ipsilateral to the infarct, they can be seen rarely contralateral to an acute stroke. A complete discussion of epileptiform abnormalities is provided in other chapters.
Periodic Lateralized Epileptiform Discharges
Periodic lateralized epileptiform discharges (PLEDs) are a relatively uncommon electroencephalographic pattern characterized by lateralized or focal periodic or near periodic spike, spike-wave, or sharp-wave complexes present throughout most of the recording. Chatrian et al. introduced the term PLEDs in 1965 [4], although the phenomenon was first described in 1952 by Echlin (5). PLEDs occur in all age groups, from infants to the aged. They are usually seen transiently in the setting of an acute destructive cerebral lesion and occur early in the course of a patient illness. They can be seen less commonly with systemic diseases and a remote cerebral lesion. Rare chronic PLEDs persisting for a period of 3 months to more than 20 years have been reported in patients with chronic brain lesions and associated partial seizure disorders. Bilateral independently occurring PLEDs were recognized by Chatrian et al. in 1965 and formally characterized in 1981. They are seen in the setting of multifocal or diffuse cerebral injuries such as anoxia and herald a less favorable prognosis with higher mortality. Approximately 80% to 90% of patients with PLEDs experience clinical seizure activity, primarily focal motor seizures (6,7).
PLEDs are often described as sharp-wave discharges coupled with additional small spikey morphological waves accompanying the larger sharp wave (Fig. 8.8). Slow waves often occur immediately after the sharp-wave discharge, creating a complex pattern that distorts the local background activity. These waves may recur at a frequency of 3 to 12 Hz per minute. By definition, PLEDs imply a lateralized process, yet this pattern can emanate from both hemispheres as in the case of bilateral PLEDs or (BIPLED) (Fig. 8.9). Although the most common cause of PLEDs is stroke, their occurrence in other conditions is frequent. In fact, CNS infections and status epilepticus predominantly account for BIPLED patterns. PLEDs have also been reported early in the Creutzfeldt-Jakob disease (CJD) and in patients with subdural hematoma, MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes), nonketotic hyperglycemia, and occasional chronic lesions, especially in the presence of a superimposed metabolic abnormality (6,8,9). Electrolyte abnormalities and nonketotic hyperglycemia have also been postulated as factors that contribute to PLEDs appearing after acute stroke (8,10).
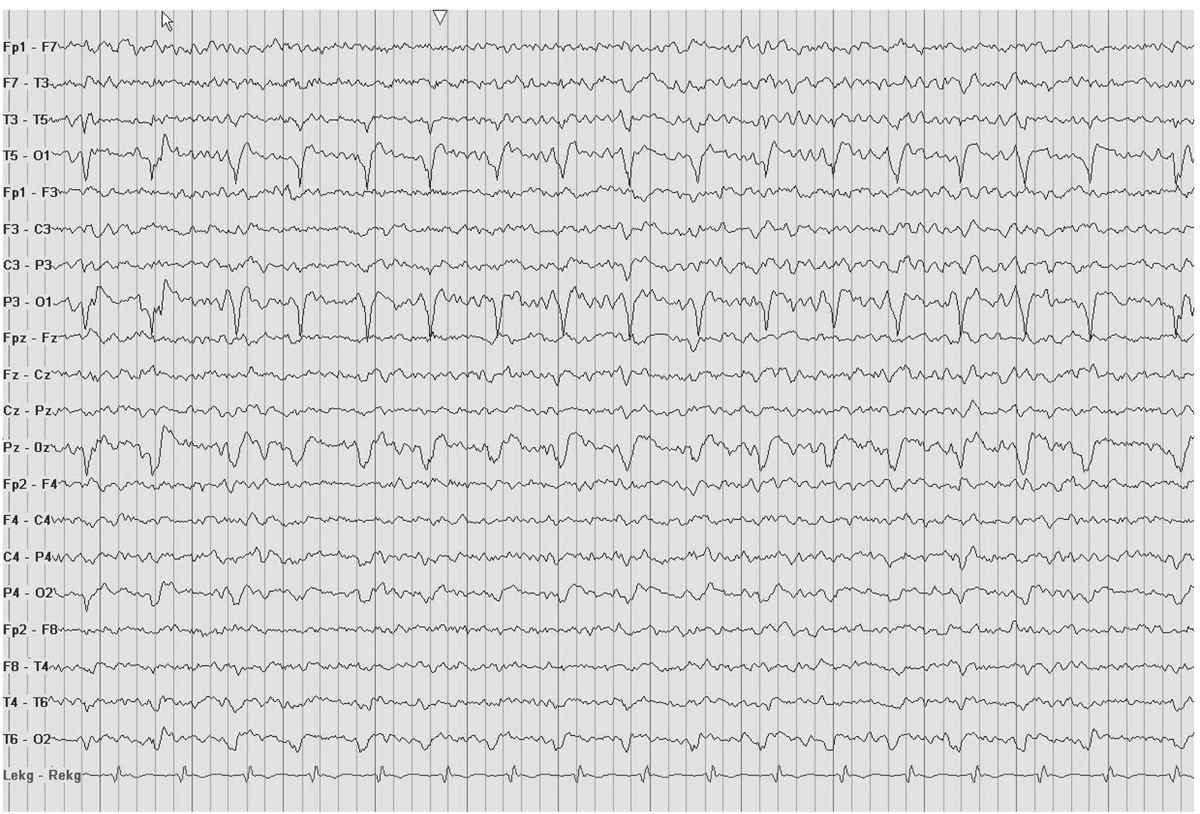
Figure 8.8: PLEDs. The EEG shows an unusual case of occipital PLEDs in a 32-year-old man with an occipital infarction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


