Economics and Cost-Effectiveness in Cardiology: Introduction
In March 2010, the United States Congress passed and the President signed historic new health care legislation, the Patient Protection and Affordable Care Act (PPACA). The full impact of this complex bill, some parts of which will not be enacted until 2014, on medical practice and US health care expenditures will likely not be clear for many years. The passage of this legislation was preceded by a vigorous public debate on the US health care system and options for its reform. Complex arguments about the best way to organize, deliver, and pay for health care became the stuff of the daily news reports and editorials. Major participants in the health care industry and other interest groups jostled to advance their perspectives before the US Congress and the court of public opinion. One major reason for all this attention and the justification given for both supporting and resisting passage of this legislation was a widespread concern about the current and future costs of health care and tremendous uncertainty about the best way to control these costs.
In 2009, the United States spent more than 17% of its gross domestic product (GDP) on health care, amounting to approximately $2 trillion.1 Approximately 50% of this expenditure went for hospital care or physician/clinical services. Drug therapy accounted for another 10%. Four major reasons can be offered to explain why health care spending keeps increasing each year: (1) innovations that improve medical care2—the care of the cardiovascular patient in 2010 is vastly different (and largely better) than it was in 1980, but innovation in diagnosis and therapy generally increases costs relative to the prior patterns of care, as is reviewed in the second part of this chapter; (2) the aging of the population—an issue for all developed countries and one that has just begun to manifest itself (the baby boomers born in 1945 reached retirement age in 2010); (3) inflation, which refers to an increase in the cost of the same good or service (contrasted with an improved medical care service described in 1, above); and (4) raised expectations of the public regarding what medicine can and should offer them when they become ill, in part due to the global information revolution created by the Internet and search engines such as Google. Patients are no longer dependent on their doctor to tell them what is possible in a particular medical situation, and they are increasingly unwilling to accept limitations in care, particularly when they are shielded from the costs by insurance.
The cost of health care is not a unique concern for the United States. Other developed nations around the globe have all experienced unwelcome upward pressure on their medical care spending. Switzerland, France, Germany, Belgium, Portugal, Austria, and Canada all spend between 10% and 12% of GDP on health care. Many of the remaining countries of the European Union as well as Japan spend between 8% and 10% of GDP on health care. The difference in spending between these countries and the United States appears due primarily to a higher rate of increase in spending in the United States since 1980. Developing nations, although spending proportionately much less on health care at present, are projected to be facing an explosion of cardiovascular and other chronic diseases over the next half-century as they develop greater affluence and their populations adopt ever more atherogenic lifestyles.3 Clearly, health care costs will continue to be a major focus of attention at the policy level and a major pressure point in clinical practice for years to come.
This chapter has two overarching goals. The first is to explain briefly what the discipline of health or medical economics is (and is not) and what useful roles it might be able to play in debates about dealing with the ever-rising costs of the medical care system. To do this, we will need to examine the sorts of questions that health economics studies and the tools it uses. The second goal of the chapter is to review some of the recent and/or important health economics literature covering specific therapies and strategies used in the care of cardiovascular patients. Emphasis here has been given to therapeutic questions relevant to prevalent cardiovascular diseases and the general cardiology or primary care practitioner. The economics of diagnostic testing is an important subject but is not covered in this chapter.
What Is Economics?
Economics is the discipline that studies how society manages and allocates its collective resources.4 Medical economics, by extension, is the study of the production and allocation of health care resources. At the risk of oversimplifying, it may be said that economics holds as axiomatic the proposition that society’s collective resources are finite and that there are many more possible uses for these resources than there are resources to be used. Consequently, choices need to be made about the best ways to use these limited (or scarce) resources to fulfill the needs and desires of society.5 The role of economics is to facilitate making these choices in an informed manner. In the health care arena, for example, health economics offers tools to study the efficiency with which different health care goods and services can produce the product of interest, improved health. In a properly functioning free market, buyers and sellers collectively determine the amount of a product that should be produced, with variations in price serving as the primary signal that connects these groups. Health care does not conform to the standard criteria for a properly functioning free market, however, and thus prices cannot be relied upon to ensure that the optimal amount of health care services to satisfy society’s wants is being produced. Large payers, professional societies, and the government all have a significant role in determining the levels of production and the patterns of distribution of health care. These interactions, as well as choices among different alternatives that require value inputs and judgments, lie outside the domain of economics, often in the realm of health and public policy.6
One other assumption that helps explain the world view of health economic practitioners is that decision makers are “rational utility maximizers.“ Utility is a concept borrowed from philosophy that refers to the amount of pleasure or satisfaction that is provided by something.7 In health economics, utilities provide a quantitative realization of the relative satisfaction felt by patients in various health states. In addition, health economics assumes that if one has two or more choices for treatment, one will always pick the one with the greater utility value. Because, in order to be satisfied, one must be alive, utilities and survival get combined to form the well-known but poorly understood metric of quality-adjusted life years (QALYs). To give a concrete example, if treatments A and B both cost $1000 but treatment A yields on average 0.1 QALYs while treatment B yields 0.3 QALYs, health economists would always expect that B would be chosen over A. In order for this to be the case, however, decision makers would need to be indifferent to who gains from these choices and who loses and such is rarely if ever the case. The “real world“ is rarely as orderly, rational, and value-neutral as it is portrayed in the economics literature.8 So health economics can perhaps be best thought of as providing simplified models of the health care economy, with the expectation that these models can provide some insights useful for decision making but are never the complete answer to any decision problem.
Comparative effectiveness is a term that has become prominent lately, and its relation to health economics is worth clarifying. Comparative effectiveness refers to research that seeks to answer important clinical questions related to real diagnostic and therapeutic options faced by significant numbers of patients.9 In short, it has the ambitious goal of developing the evidence needed to practice medicine. This is to be contrasted, perhaps, with evidence that is developed to seek regulatory approval for marketing of new products from the US Food and Drug Administration (FDA), where the comparator therapy in a pivotal trial might be “placebo.“ It can also be contrasted with evidence developed in very focused, optimized clinical trials that seek to demonstrate a proof of concept.
Some have proposed that comparative effectiveness research will serve as a counterweight to health care policy decision making that seeks primarily to maximize efficiency/minimize costs.10 One provision of the 2010 PPACA is to establish a Patient-Centered Outcomes Research Institute as a nonprofit organization independent of the federal government. The institute is advisory in that none of its findings about comparative effectiveness bind the Secretary of Health and Human Services to cover or not cover specific types of medical care. Further, this new institute is specifically prohibited from using cost effectiveness as a threshold to establish what types of care are recommended or reimbursed. The role of cost and economic considerations in comparative effectiveness research varies according to the source consulted. In the case of this legislation, high sensitivities about the possibility of the federal government rationing care as a way to save money resulted in a de-emphasis of the role of economics. However, there is not really any inherent tension between comparative effectiveness and high-quality health economics work, because both always start with the clinical comparative effectiveness questions before reaching any cost questions. Thus, it may be useful to consider health economics research a comprehensive form of comparative effectiveness research.
The Tools and Concepts of the Health Economist
As in other clinical research disciplines, the most important tools of the health economist are (1) formulating appropriate insightful questions, (2) assembling the highest quality data possible that is relevant to these questions, and (3) analyzing the data using methods that are transparent and appropriate to the questions under study.
Although it is natural to expect that the most important questions for a health economics analysis would deal with some aspect of cost, understanding the clinical effectiveness is actually almost always much more important. The first question of importance regarding a particular health care service or technology is: “What long-term effects (both positive and negative) does it have on health outcomes and what is the level of evidence supporting the current state of understanding about clinical effectiveness?“ Without a clear answer to this question, no economic analysis can be of much use. Sometimes the most important service an economic analysis can perform is to focus a bright light on the fact that the answer to this seemingly simple question is not at all clear. It is not uncommon to encounter a model-based economic analysis in the published literature where the authors have reached some conclusion about whether or not a given medical intervention is cost effective, yet the clinical literature is not at all settled even on what effects that intervention produces. Because of the critical importance of this issue, the second portion of this chapter includes many references to the relevant evidence base supporting clinical effectiveness as an integral part of the process of addressing the topics of cost and cost effectiveness.
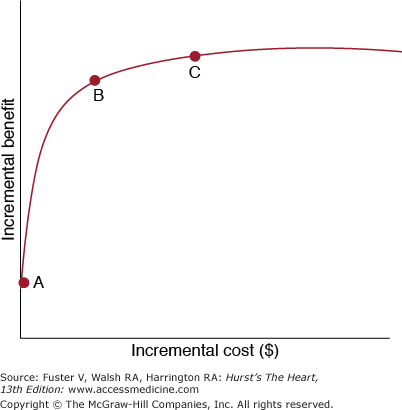
The second critical health economics question is: “Is it good value for the money?“ By good value, we basically mean that a given health care service or product can be used to produce a relatively large amount of “health“ for relatively little money (Fig. 112–1). Another way to phrase this question is: “How efficiently does this new therapy or strategy we are studying produce extra units of health (sometimes expressed as QALYs)?“ Answering this question requires that we combine our best understanding of the long-term clinical effectiveness with our best understanding of the long-term cost effects of the intervention in question. To do this, one of three possible economic efficiency analysis methods are often used (Table 112–1). These methods differ primarily in the way in which the health benefits are expressed in the analyses. Cost-benefit analysis requires all incremental health benefits to be converted to their monetary equivalent. As might be imagined, the methods for doing this are controversial because they require answers to some of society’s most difficult value questions. Although it may be possible, for example, to assign monetary value to future life years for a woman or man who does not work outside the home by calculating what it would cost to hire someone to do the same in-home tasks performed by that individual, valuing the survival of subjects with disability or subjects who are retired is more difficult. Grandfather may be a valued member of the family, but how should society assess the value of his life years once he stops working? Cost-effectiveness analysis avoids this hornet’s nest of difficulty by valuing all units of survival the same regardless of who benefits. Cost-utility analysis modifies this by using utility values (patient or societal preference weights) to adjust the survival data. Thus survival in a severely disabled state or in severe pain would be valued significantly less (lower utilities and thus lower calculated QALYs) than survival in good or excellent health. Although the notion of utility might seem like a good solution to the difficult problem of valuing on a common scale all the types of health outcomes produced by the health care system, considerable difficulties remain unresolved.11 For example, who is best equipped to decide the utility weight of a year with a severe stroke or a year with severe heart failure? Should it be the member of the general public whose primary encounters with the medical system have been in the form of annual physicals? How much information about the health states being ranked do such individuals need in order to make informed judgments? Should it be health care professionals who are most familiar with the outcomes of these patients? Should it be the patients themselves? Two specific difficulties present themselves in the last option. First, the severe stroke patient may not be able to participate in any assessment process. Second, no matter how severe or unpleasant the health state may seem to an outsider, humans have a remarkable ability to adapt and to accommodate to their disabilities and limitations (sometimes referred to as hedonic adaptation), and so their own valuation of their health state may be considerably higher than that given by a member of the general public. Cost-effectiveness analysis provides an estimate of the relationship between money spent and health benefits produced, but unlike cost-benefit analysis, the cost-effectiveness and cost-utility ratios have no natural interpretation. Thus benchmarks are needed to help understand the results of these analyses. Typically in the United States, a cost-effectiveness ratio of $50,000 or less per life year (or QALY) saved is considered “economically attractive,“ values of $100,000 or more per life year (or QALY) saved are “economically unattractive,“ and the range between these two zones is of uncertain economic attractiveness. The $50,000 figure was apparently initially chosen because it was the cost to keep an end-stage renal failure patient alive on hemodialysis for a year relative to no dialysis. Dialysis is a form of medical care that Congress had guaranteed to fund for all affected renal failure patients and thus represents a de facto policy statement by the US federal government on its willingness to pay to save a life year. One criticism of this benchmark is that it has not been updated in more than a decade. A recent evaluation of 13 studies published between 1968 and 1998 on the cost of renal replacement therapy found that the cost of center dialysis was between $55,000 and $80,000 per life year saved.12 A more recent analysis of this issue using a computer simulation model and data from 1996 to 2003 found that renal dialysis had a cost per QALY of $129,000.13 The $50,000 per QALY benchmark may therefore be too conservative given current decision making on paying for medical care.14 Other countries use different, more conservative benchmarks. For example, the National Institute for Health and Clinical Excellence (NICE) in the United Kingdom uses a benchmark of less than or equal to £30,000 per QALY to define economic attractiveness, and there has been recent discussion in the United Kingdom to lower this threshold to £20,000 per QALY.15
Figure 112–1
This graph shows the relationship between investment of incremental health care resources and the resulting incremental health care benefit. A represents a point where large health benefits are generated at low cost. C represents a point where vanishingly small health benefits are realized despite large investments, also known as “flat of the curve“ medicine. B represents a cutpoint, or benchmark, beyond which therapies are no longer considered economically attractive.
| Strategy | Treatment Costs | Effectiveness (Life Years Gained) | Utility (QOL) | QOL-Adjusted Life Expectancy | Benefitsa |
|---|---|---|---|---|---|
| Treatment A | $30,000 | 3 | 0.79 | 2.37 QALYs | $5000 |
| Treatment B | $15,000 | 2 | 0.84 | 1.68 QALYs | $3000 |
The third major question of health economics is: “Can we afford it?“ This question really represents the intersection of policy and health economics. Public policy is concerned with the large decisions about how much of society’s resources to devote to producing more health care. In the United States, this is defined both by the amount of funding provided to Medicare (primary public funder of health care) by Congress and the health care investment and reimbursement policies in the private sector. Economics can then address the question of what the aggregate cost to society or to individual payers will be from funding different diagnostic and management strategies. In Canada and much of Europe, where health care spending is mostly controlled by central governments, the effect of new therapies and tests on the health care budget is always an issue, and reimbursement policies are carefully crafted to control dissemination. In the United States, Medicare is prohibited from considering cost in coverage decisions and the lack of a single central payer means that funding decisions get made in a more fragmentary and disjointed way. However, even with central decision making, funding decisions are not necessarily made to optimize health production. One major obstacle is that it is much easier in such a system to say no to some new innovation than to stop or reduce funds for some existing therapy because it is not evidence-based or because it is now a less efficient method of producing more population health.
As with any form of empirical research, the value of economic analysis is highly dependent on the quality and relevance of both the clinical and economic data available for the work. Ideally, data at the patient level can be obtained on both clinical outcomes and resource consumptions/costs from the same cohort. However, even this condition does not ensure the best quality. For example, a pivotal guideline-changing clinical trial may provide excellent clinical outcomes data but have almost no useful resource use or cost information. Estimating costs from the few possibly relevant variables in such a data set may be the best that can be done, but the limitations of this should not be overlooked. Many examples can be found in cardiovascular medicine. Alternatively, a small clinical trial may provide detailed economic data, but its clinical efficacy data is not reliable due to the sample size.
When we refer to “economic data,“ generally we mean resource use information and associated costs. Specifically, economists refer to the incremental resources needed to produce a good or service, by which they mean only the extra resources required. If we are comparing a new treatment with standard care, for example, and both of them will involve 5 days in the hospital at the same level of intensity of care from doctors and nurses, then there is no incremental hospital stay involved in the new treatment. Incremental costs then are the monetary value of the incremental resources consumed. The primary emphasis on resource consumption rather than money is deliberate. Money is a tool that facilitates the functioning of markets, so that barter is not required. The meaning of money is always traced back to the resources consumed. Economists refer to opportunity cost to designate what was given up or lost to society when a decision was made to produce something else. If we decide to produce 10,000 more coronary bypass surgeries in the United States, for example, we will lose the resources consumed in that process to alternative uses. In the world of limited or scarce resources referred to earlier, it is this sort of trade-off that underlies the economists’ fundamental notion of cost.
Classical economics further maintains that in a well-functioning free market, the equilibrium price of the good or service in question represents its opportunity cost. Because the medical marketplace violates a number of key conditions required to qualify as a well-functioning free market, the economic meaning of medical prices is problematic. Costs that reflect the production process of the medical care are a reasonable surrogate for economic value. However, the charges that are contained on US medical bills are completely unreliable for economic analysis purposes, and the reimbursements paid to hospitals and physicians are typically less suitable for this use outside of very specific questions relevant to payers or providers. Methods for estimating medical costs have recently been reviewed.16,17
Induced costs/savings are the costs and cost savings that occur consequent to some treatment or strategy used in patient care. For example, use of thrombolytic therapy for ST-segment elevation acute myocardial infarction (MI) would entail the initial cost of the drug (for tenecteplase, >$2000). If the patient in question had an intracranial hemorrhage, the extra costs of caring for that complication would be induced costs, and clearly those costs would most likely extend for the rest of that patient’s lifetime. If, instead, treating the patient during the first hour of her large anterior MI prevented her from developing debilitating heart failure later, then all the money that would not have to be spent over the ensuing years of her life caring for that complication would need to be counted as an induced cost savings. In an analysis that extends many years into the future, future costs and savings need to be discounted (typically at 3% per year) to calculate the present value of the lifetime cost stream. The reason is straightforward. We are not indifferent to the prospect of receiving $100 today or receiving the same amount in 10 years. We recognize that in 10 years that money will be worth less to us than it is today, because we could have had it in the bank earning interest for 10 years if we had received it today. Thus discounting is a way of reducing the nominal value of future spending and savings to their present value equivalents.
A further complexity about the concept of cost is that a perspective must always be defined in order to measure and understand costs. For example, if a patient undergoes a successful percutaneous coronary intervention (PCI) with a drug-eluting stent and is discharged from the hospital without complications but is readmitted 3 months later with a stent thrombosis, that readmission is a cost from the payer’s perspective and from the societal perspective but not from the hospital’s perspective or the physician’s perspective unless they are operating in a capitated system. If a patient comes to clinic with chest pain and gets a clinical workup and a stress test, the cost of all that from the patient’s perspective (assuming they are insured) may simply be his co-pay. The clinic and society, however, each have different perspectives on that same episode of care. In most economic analyses, the primary analyses should be done using the societal perspective, which is the perspective in which the winner/loser problem described in the above examples is minimized.
The problem of measuring and comparing costs across international borders is a difficult one that offers no easy solution but is increasingly important in the current era of international cardiology mega-trials.18,19 To the extent that practice patterns are converging around common standards that cross international boarders, it may make sense to pool resource use data across boarders and assign common costs. However, local distortions in price data make it problematic to decide what prices to use in such an exercise. Further, there is still good evidence that local practice patterns differ substantially in different regions of the world. For example, length of stay after acute MI and the rate of referral for invasive angiography vary considerably across countries.20
Several different types of models are now commonly used in health economics. Descriptive and predictive statistical cost models are stochastic mathematical models that treat cost as a probabilistic quantity. Although that might sound quite technical and arcane, these models are quite analogous to descriptive and predictive regression models of clinical outcome variables. Such models might be developed to understand the major clinical correlates of cost in a particular cohort or to be able to predict the cost of future patients with particular clinical features treated in a specific way. To develop these sorts of models, one needs patient-level data—ideally for both clinical and cost descriptors of interest. More complex models based on regression methods are also now commonly used in health economics. For example, multilevel (sometimes called hierarchical) models can be used to explore patient level, provider level, institutional level, and even country level influences on cost and outcomes. High-quality patient-level data and regression modeling techniques can be used to estimate both lifetime costs and lifetime clinical outcomes in a cost-effectiveness analysis.
These patient-level models can be contrasted with deterministic models used in health economics, including decision models. These latter models have been most often used to estimate lifetime costs and outcomes for cost-effectiveness analysis. Typically, they do not use patient-level data but instead employ group level summary data obtained from various (unrelated) published sources. Their strength lies in providing a framework for combining such disparate data estimates into a summary model focused on a specific question. Because these data do not incorporate any measure of the variability in the estimates, the resulting models are often interpreted as showing what will happen (deterministic) rather than what might happen (probabilistic or stochastic). The simplest decision models employ basic probability calculations to estimate the (uncertain) outcomes from each strategy or therapy being studied. More complex methods of handling the uncertainty in such models includes the use of Markov models (also called state transition models), where the probability of moving from one health outcome state to another in each time cycle of the model (eg, 1 month or 1 year) is represented as an explicitly specified transition probability. The limitations to incorporating more of the nuances of clinical practice in such models are that the models become quite complex to manipulate and to explain, and also that the data sources available to populate the model may not be sufficient to support the complexity the analyst desires to examine.
One major reason that so much modeling is done in health economics is that clinical studies, even the best ones, rarely provide all the data that is needed for a comprehensive health economics analysis. For most analyses, in fact, the time frame for a health economics analysis is the full time span during which some residual effect of the testing or treatment strategy being studied might be expected. For most clinical problems involving patients with a chronic disease such as the ones discussed in the second part of this chapter, the lifetime of the patient cohort is the relevant timeframe of interest. If full stream of costs and outcomes over this timeframe could be observed and measured, no modeling-based extrapolation would be required. Because for adult chronic diseases this is almost never possible, some amount of model-based extrapolation is required. The question of how best to do that has no simple answer, and different authorities in the field favor different approaches. Because the models are quite difficult to validate empirically, the matter of which approach is to be preferred is not readily discovered.
Given this state of affairs, the consumers of these models can best arm themselves for forays into this literature by keeping in mind a few basic questions. First, are the assumptions clear? All models require assumptions, and in some cases the assumptions are quite important in deciding how much credence to give the results. Second, are the important cost and outcome inputs clearly specified? Third, are the major moving parts of the analysis sufficiently transparent for readers to understand the final results? The model should not be presented simply as a black box where data goes in one end and an “answer“ emerges from the other. Health economics is not discovery science. A cost-effectiveness model, therefore, is not a method of uncovering new medical insights but rather should provide a clearer understanding between money spent and health outcomes produced for that money. Finally, are the key uncertainties in the analysis fully explored via stochastic and sensitivity analysis methods?
The primary concern of economics is choosing among alternatives. Thus economic efficiency metrics such as a cost-effectiveness ratio must be expressed in terms that are common to all the alternatives of the choices to be made. When analysts want to be able to compare medical investment choices with choices in other sectors of the economy such as education, transportation, and defense, it is often necessary to use cost-benefit analysis because there is no other common method of expressing the outcome being produced by the investment being considered. However, when all choices reside in the health care arena, cost-effectiveness and cost-utility analysis are much more commonly used, with the most common effectiveness metric being the added life year or the QALY. In such circumstance, from a cost-effectiveness analysis perspective, all medical outcomes must be translated into their effect on length and quality of life. When dealing with an intermediate outcome such as a change in a biomarker or a change in the degree of stenosis of a coronary artery, this requirement may seem overly burdensome. However, using therapies that have both risk and cost to alter biomarkers or coronary artery appearances without some reasonable expectation that the patient will benefit over the long term either in terms of length or quality of life is hard to justify on a number of different grounds. And if there is a belief that these outcomes are benefited but the amount is uncertain because of a lack of appropriate clinical data, then that uncertainty is an important part of the story about the therapy or management strategy being studied.
The pattern by which quantity and quality of life are altered by therapies can vary in several different ways. For example, all the incremental outcomes differences may accrue early (eg, by 30 days), they may accrue steadily in a linear fashion over time, or they may even amplify over time. The pattern of cost differences over time also can vary in different circumstances and does not have to mirror the pattern of incremental outcome benefits. Cost differences may be large or small, may accrue early, steadily over time, or as a step function. For example, surgical therapies such as coronary artery bypass graft surgery (CABG) and heart transplant have very high upfront costs, and the initial clinical benefits may even be negative due to operative mortality. Outcome benefits may take years to be fully expressed in such cases so that the incremental outcomes calculated from short-term follow-up (eg, 1 year) are quite likely to underestimate such benefits. Secondary prevention, on the other hand, often involves a steady cost year after year and in some cases a small clinical benefit that tends to amplify over time. The key point that these examples serve to highlight is that a high-quality economic analysis must assess outcomes and costs over the long-term, defined operationally as the period for which the effects of the therapy in question, both direct and induced, might reasonably be expected to persist.
A few points that have been made earlier in this chapter are worth emphasizing because they are so central to understanding the perspective of health economics. First, the focus is typically on systems, countries, or society and not on individual doctors or patients. Health economics, then, does not usually focus on decision making at the level of the individual patient, nor is it intended that individual physicians should be using these methods to make individual “funding decisions“ about what care to provide to patients.
Second, health economics is neutral on the issue of who benefits and who is deprived of health care services. If it were possible to generate more QALYs by treating an 80-year-old patient with advanced heart failure than a 30-year-old with hypertension or a premature infant with respiratory distress, and only one could be chosen, health economics would favor going the way of more efficiency. Clinicians usually do not face such overt choices, although triage situations do come close, as when coronary care unit beds or donor hearts must be allocated and there are more candidates than resources to satisfy all who could benefit. Health economics in this context is a tool that may clarify some important issues about the choices being considered. However, societal values and preferences may and often should override the maximal efficiency answer.
Finally, although clinicians are now accustomed to using fairly rigorous standards to decide whether new therapies and clinical strategies “work“ and practice medicine using guidelines that rank recommendations according to the strength of the evidence, they may not appreciate that policy decisions are often made on the basis of “evidence“ that would not pass rudimentary quality screens in medical/outcomes research. Thus clinicians who are informed, discerning consumers of health economics research will be critical to help drive comparative effectiveness research toward higher quality rather than political expediency.
Selected Health Economics Applications
(See also Chaps. 26, 27, 28, 29, 30.) According to statistics compiled by the American Heart Association, approximately 5 million people in the United States have heart failure (the prevalence) and every year 550,000 new cases are diagnosed (the incidence).21 In the Medicare population (age ≥65 years), heart failure is the most common reason for hospitalization listed on medical claims data. In the total US population, the estimated number of annual heart failure hospitalizations approaches 1 million a year. Further, patients who are hospitalized for this diagnosis have a substantially elevated risk of being rehospitalized for the same diagnosis within the following 6 months. The overall annual US medical spending attributed to heart failure is approximately $39 billion.22 It must be kept in mind that given the difficulties in identifying heart failure on a population level, these estimates are extremely crude. However, if we accept the reasonable contention that heart failure is common and accounts for a large amount of health care spending, it follows to reason that if we could allocate resources to prevent the disease or, failing that, prevent it from progressing to decompensation, we might identify opportunities to improve the health of these patients efficiently and without a massive increase in health care spending. Heart failure prevention means better control of the disorders most likely to progress to heart failure, primarily coronary artery disease (CAD) and hypertension. Additional consideration of these strategies will therefore be found in those sections of this chapter. In the remainder of this section, we will examine three general types of care used to manage the heart failure state: maintenance medical care to relieve symptoms, improve functional status, and improve prognosis; strategies to reverse the heart failure state and to prevent sudden cardiac death; and comprehensive management strategies to improve functioning and reduce acute decompensations.
Medical therapy of heart failure focuses on symptom relief and improvement of prognosis. As reviewed elsewhere in this text, the main agents shown to enhance survival are angiotensin-converting enzyme (ACE) inhibitors, β-blockers, and angiotensin receptor blockers (ARBs), as well as isosorbide-hydralazine and aldosterone antagonists in selected populations. Unfortunately, much of the literature examining the economics of these therapies is now either dated or most pertinent to a non-US health care system. In addition, few of the pivotal trials that changed our clinical practice included careful prospective measurement of resource use and costs, so most of the literature is based on decision modeling frameworks.
In one such analysis, Glick and colleagues23 examined the cost effectiveness of enalapril using data from the SOLVD Treatment trial, which randomized 2569 symptomatic heart failure patients with left ventricular ejection fraction ≤0.35 to receive either enalapril or placebo. Over a projected 7-year life expectancy, these authors estimated that enalapril would add 0.3 discounted life years (0.21 discounted QALYs) and would have a net lifetime incremental cost of $25, with a cost-effectiveness ratio of $80 per life year added ($115 per QALY added). Given that generic enalapril (along with lisinopril and benazepril) can now be obtained in the United States and that the Glick analysis probably underestimated the long-term effects of therapy on reducing hospitalizations, it is very possible that enalapril as used in SOLVD Treatment trial was cost saving over the long run.
Three β-blockers have been shown to improve survival in heart failure patients when added to ACE inhibitors: sustained release metoprolol, bisoprolol, and carvedilol. The clinical data show that these drugs also reduce all-cause hospitalization, although the magnitude of this effect varies among the trials. Carvedilol is now available in a generic form. The US Carvedilol Heart Failure Program, consisting of four trials, was used as the basis for a Markov decision model analysis, combining the trial data with varying assumptions about what would happen to benefits as the trial data were projected to a lifetime time horizon.24 Life expectancy without carvedilol was estimated at 6.7 years, and carvedilol therapy was projected to add 0.31 to 0.95 life years at an incremental lifetime cost (1999 costs) of $7600. Given the difference between proprietary drug costs used in that analysis and current generic costs, carvedilol would now be expected to have a cost-effectiveness ratio much more favorable than the $30,000 per life year calculated in this older model. Bisoprolol is also available in generic form, but the trials supporting this drug were done in Europe, and no high-quality economic data are available for the United States. Similarly, no US economic analysis has been done of the extended-release form of metoprolol succinate used in the MERIT-HF trial.
ARBs improve survival and reduce cardiovascular hospitalizations in heart failure patients unable to tolerate ACE inhibitors,25,26 but their benefit as a first-line treatment in place of or as an adjunct to ACE inhibitors is less clear.27–29 There are no prospective economic analyses from a US perspective of the major clinical trials comparing ARBs with placebo or with ACE inhibitors. An economic analysis from a European perspective of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program found the use of candesartan to be economically efficient compared with placebo in heart failure patients with an ejection fraction ≤0.40, with candesartan achieving economic dominance (better clinical outcomes and lower costs) in some scenarios.30 Although there are currently no generic ARBs approved for use in the United States, the patents are scheduled to expire on several proprietary ARBs in the next 2 years, and this will likely result in less costly generics.
The isosorbide-hydralazine combination studied in the African-American Heart Failure Trial (A-HeFT) reduced heart failure hospitalizations during the study’s mean 13-month follow-up (from 0.47 per patient to 0.33) and reduced total medical costs during the study follow-up by approximately $4300, exclusive of drug costs.31 Even with the assumed drug cost per day of $6, the isosorbide-hydralazine combination was economically dominant. Because the specific proprietary combination drug studied is no longer available, the cost of these two drugs would now be significantly less, reinforcing the likelihood that this treatment is very economically attractive for the population studied in A-HeFT.
Once the mainstay of heart failure treatment along with diuretics, digoxin is now considered in patients in atrial fibrillation and in patients not responding adequately to the other agents discussed in this section. The data from the Digitalis Investigation Group (DIG) trial showed that although this drug did not affect survival, it did reduce hospitalization rates by 6%. A recent economic analysis of the trial found that the net cost of digoxin therapy over 3 years was approximately $160.32 Although the reduction in hospitalizations might have expected to produce a net cost-saving result, the digoxin arm had higher non–heart failure–related costs, including more balloon angioplasties, an observation that remains unexplained.
Two aldosterone antagonists have been studied in selected populations with heart failure, spironolactone in the Randomized Aldactone Evaluation Study (RALES) and eplerenone in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). A decision model–based analysis looking at the first 35 months of the RALES trial found that spironolactone added 0.13 QALYs and had a net lower cost than the placebo arm.33 In a lifetime analysis of the EPHESUS trial results, eplerenone added approximately 0.10 to 0.13 life years and had a net cost of approximately $1400 due to the drug cost (in this analysis ∼$3.60 per day).34 The cost-effectiveness ratio, using several different data sets upon which to base the long-term extrapolations, was consistently under $20,000 per life year added.
One interesting analysis examined the hypothetical effect of increasing the use of ACE inhibitors, β-blockers, spironolactone, and digoxin each by 10% in a Canadian cohort of 86,000 patients with heart failure and estimated that, after accounting for the extra cost of the drug therapy ($4.3 million in Canadian prices), this strategy would save $2.3 million net in the first year due to reduced avoidable hospitalizations.35
(See also Chaps. 29 to 30.) For patients with advanced refractory heart failure, cardiac replacement therapy may offer the best option to restore quality of life and improve longevity. The economics of orthotopic heart transplant have not received adequate economic study in recent years. Approximately 10 years ago, the estimate of overall costs for heart transplant in the United States was approximately $700 million.36 The reason this figure was not higher is that the supply of donor hearts for transplant has remained limited to approximately 2000 per year, with no signs this number is likely to increase. In one US academic center, the initial costs of a transplant from the time of the operation to discharge averaged approximately $150,000.37 In another academic center, transplant patients averaged 2.1 hospitalizations and 11.9 outpatient encounters during the first posttransplant year.38 Contemporary annual posttransplant costs can total more than $70,000 per patient due in part to the need for expensive immunosuppressive drugs and the need for complex hospitalizations to treat rejection episodes and infections. Despite its high procedural and follow-up costs, the substantial increase in life expectancy (one of the largest of any cardiovascular therapy) and improvement in quality of life for transplant patients makes it likely to be economically attractive compared with medical therapy alone in this population.
Because left ventricular assist devices (LVADs) are not limited in number the way donor hearts currently are, the potential exists for this form of cardiac replacement therapy to have a much larger clinical and economic impact. The technology and ancillary care has evolved significantly since the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial. A recent systematic review of the economics of LVAD identified 18 studies reporting costs but only 4 examining cost effectiveness and only 1 from the United States.39 Although LVAD therapy is quite expensive initially, with device costs alone in excess of $70,000, the therapy has the potential to be cost effective if, like heart transplant, it provides a significant enhancement of survival and/or quality of life. However, a recent review of Medicare patients receiving VADs provides less than clear answers on this point. In 1476 patients who received an LVAD as first or primary therapy (2000-2006), hospital survival after implantation was 69% and the 1-year survival was 52%, which is significantly worse than that after heart transplant.40 Of those discharged alive with a device, 55% had at least one readmission within 6 months. Mean Medicare payments at 1 year for inpatient care averaged $179,000. These data all suggest that destination VAD therapy is currently in a transition state, as clinicians attempt to understand the population most likely to benefit from these devices.
Another quite different approach to the treatment of patients with advanced heart failure derives from observations that selected patients who have severe left ventricular dysfunction accompanied by significant interventricular conduction delay or bundle-branch block have very inefficient ventricular conduction patterns due to their dyssynchronous pattern of depolarization and contraction. In such patients, the use of biventricular pacing for cardiac resynchronization has been shown to improve heart failure symptoms (by ∼ one New York Heart Association [NYHA] class) and to improve survival. An economic analysis of the Cardiac Resynchronization in Heart Failure (CARE-HF) trial done using UK cost weights found a cost-effectiveness ratio for cardiac resynchronization therapy (CRT) plus medical therapy of €19,319 per QALY and €43,596 per life year compared with medical therapy alone, indicating the important contribution of quality-of-life improvements to the estimated cost effectiveness in this trial.41
In Europe, many CRT devices are implanted without implantable cardioverter defibrillator (ICD) function (CRT-P), whereas in the United States, most of the CRT devices include an ICD function (CRT-D), a factor that influences the cost of the device. The incremental contribution of the ICD function versus the CRT function in these patients to the survival benefits seen remains unsettled. An economic model based on the US Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial data found that with extrapolation to 7 years, CRT-P had a cost-effectiveness ratio of $19,600 per QALY relative to medical care alone and CRT-D had a cost-effectiveness ratio of $43,000 per QALY relative to medical therapy alone.42 What was left unaddressed by the COMPANION trial is the incremental benefits and cost effectiveness of CRT-D relative to CRT-P.
The Multicenter Automatic Defibrillator Implantation Trial (MADIT)-CRT trial examined CRT-D versus ICD alone in 1820 NYHA class I or II heart failure patients (both ischemic and nonischemic) with an ejection fraction ≤0.30 and a QRS duration of 130 ms or more and found the addition of CRT to ICD therapy reduced heart failure events by 41% (22.8% for ICD-only group vs 13.9% for ICD + CRT; P < .001).43 No effect was seen, however, on all-cause mortality. An economic analysis from this trial is currently under preparation.
(See also Chap. 49.) The MADIT-II trial and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) established that ICD therapy improves survival over medical therapy alone in patients with reduced left ventricular ejection fraction.44,45 A prospective economic analysis of the MADIT-II trial found ICD therapy in patients with reduced left ventricular ejection fraction after MI to have an estimated incremental cost-effectiveness ratio of $235,000 per life year saved based on an average survival gain of 0.167 years after 3.5 years of follow-up.46 However, this analysis made the conservative assumption for the base case analysis that no further survival benefits would accrue from ICD therapy beyond the empirical follow-up period of the trial. Projection to 12-year follow-up estimated the cost-effectiveness ratio to range from $78,600 to $114,000. Empirical 10-year follow-up from MADIT-II showed that survival benefits from ICD therapy continued to amplify over time, indicating that the initial economic analysis from this study was too conservative. A separate economic model based on MADIT-II eligibility criteria found the estimated cost-effectiveness ratio in this patient population varied depending on follow-up time horizon, from $367,200 at 3 years to $67,800 at 15 years.47
A prospective economic analysis of the SCD-HeFT trial using a lifetime time horizon found a more favorable cost-effectiveness ratio for ICD therapy compared with medical therapy alone in stable, moderately symptomatic heart failure patients with a left ventricular ejection fraction ≤0.35. In this economic analysis, the base case lifetime cost-effectiveness ratio was $38,389 per life year saved and $41,530 per quality-adjusted life year saved.48 Similar to the aforementioned economic analyses, the SCD-HeFT investigators estimated the cost-effectiveness ratio would improve with extended follow-up, with $127,503 per life year saved at 5 years and $58,510 per life year saved at 12 years. However, in subgroup analyses, ICD therapy in patients with NYHA class III heart failure was found to increase costs without an incremental benefit and thus was dominated by medical therapy alone. Ten-year follow-up of this study is underway.
A Markov model using data from several clinical trials of ICD therapy was constructed to estimate the cost effectiveness of ICD therapy under different assumptions.49 This analysis found that prophylactic ICD therapy added between 1.01 and 2.99 quality-adjusted life years at a cost of $68,300 to $101,500 in patients with left ventricular systolic dysfunction. The incremental cost effectiveness of ICD therapy in this analysis ranged from $34,000 to $70,200 per QALY, with ICD therapy remaining below the upper cost-effectiveness benchmark of $100,000 per QALY if the incremental survival benefit of ICD therapy lasted ≥7 years.49
Several meta-analyses of heart failure disease management programs have been published, each using a somewhat different set of trials and reaching somewhat different conclusions. In one report using 19 trials involving 5752 patients, disease management produced a significant decrease in all-cause hospitalization.50 In a second analysis of 36 studies including 8341 patients, disease management produced a 3% decrease in mortality and an 8% decrease in rehospitalization.51 Both meta-analyses found significant heterogeneity among individual trials. A third overview found that in 15 of 18 trials, disease management was cost saving. This study also found that follow-up of patients by a specialized multidisciplinary team reduced mortality, whereas other configurations of disease management reduced hospitalizations but not mortality.
(See also Chap. 40.) Atrial fibrillation (AF) affects between 2.3 and 2.7 million Americans, and approximately 75,000 new cases are diagnosed each year.22,52 AF is associated with a shorter life expectancy and it adversely impacts patient quality of life, increases the risk of cardiovascular events, complicates heart failure, and significantly increases the likelihood and severity of stroke, all of which are associated with substantial medical costs.
Because of its co-occurrence with common cardiovascular diseases such as CAD and heart failure, determining the unique economic burden of AF per se is difficult. However, a retrospective analysis of data from three federally funded US databases found that AF, either directly or as a comorbidity, accounted for 350,000 hospitalizations, 5 million office visits, 276,000 emergency department visits, and 234,000 outpatient department visits in the year 2001, with a cost of more than $6.6 billion.53 A more recent estimate for the year 2006 places AF hospital discharges at 461,000.22 Additionally, 15% to 20% of ischemic strokes (∼ 140,000 per year) are believed attributable to AF, with associated costs exceeding $9 billion.22 None of the current AF therapies targeted at mitigating symptoms and preventing stroke or tachycardia-induced cardiomyopathy are known to reduce mortality in AF patients.54 Thus reducing resource use and improving patient quality of life are the main factors impacting economic analyses of contemporary AF therapies.
Warfarin therapy has been found to reduce the risk of stroke in AF patients by 62% (compared with a 22% risk reduction with aspirin) and is recommended in current practice guidelines. Unfortunately, the efficacy of warfarin therapy in preventing strokes is tempered in practice by its variable anticoagulation effects and associated risk of bleeding. For an optimal risk-benefit ratio with warfarin therapy, frequent monitoring must be performed to ensure that the international normalized ratio (INR) is maintained within the target therapeutic range. Such monitoring is particularly important in patients at high risk for bleeds, such as elderly individuals. However, in clinical practice, regular monitoring poses logistical difficulties and increases the cost of care, thereby increasing the likelihood of poorly controlled anticoagulation. Despite these difficulties, warfarin is highly effective, and its generic price for maintenance therapy is less than $1 per day in the United States and pennies a day in European countries, which makes it very efficient from the economic perspective. Strategies to overcome warfarin dosing challenges have shown mixed results. Whereas an anticoagulation management service may provide greater effectiveness while reducing costs compared with usual care,55 genotype-guided warfarin dosing for AF patients does not appear to be cost effective at this time.56,57
Dabigatran is a novel direct thrombin inhibitor that, at a 150-mg dose twice a day, has been shown to be superior to warfarin at reducing stroke or systemic embolism in AF patients (relative risk = 0.66; 95% confidence interval, 0.53-0.82; P < 0.001), with both treatments showing similar rates of mortality and major hemorrhage after 2 years of follow-up.58 Dabigatran is not FDA approved at the time of this writing, and thus estimating its cost effectiveness compared with warfarin is not possible for the US health care system. To date, studies of dabigatran have not shown the potential for liver toxicity that halted the FDA approval of another direct thrombin inhibitor, ximelagatran. A Markov decision model of ximelagatran in a cohort of 70-year old patients with chronic AF found that for the drug to achieve a cost-effectiveness ratio less than $50,000 per QALY relative to standard warfarin therapy, ximelagatran would have needed to cost less than $1100 per year or be prescribed to subsets of patients with very poor quality of life with warfarin.59
Another medical therapy option for stroke prevention in AF patients is clopidogrel alone or in combination with aspirin. In AF patients with one or more additional risk factors for stroke, the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) W trial investigators found warfarin to be superior to clopidogrel plus aspirin at preventing vascular events, thus negating any need to determine the economic attractiveness of clopidogrel compared with warfarin in this patient population.60 The ACTIVE A trial investigated clopidogrel alone versus clopidogrel plus aspirin in AF patients who could not tolerate vitamin K antagonist therapies. Clopidogrel plus aspirin was superior at reducing a combined end point of major vascular events including stroke, MI, systemic embolism, or death from vascular causes, but this benefit came with a significantly increased risk for major hemorrhage.61 The economic attractiveness of this strategy has not yet been assessed.
The two primary approaches to managing AF are establishing and maintaining sinus rhythm (rhythm control) and controlling ventricular rate (rate control). Despite their conceptual appeal, rhythm control strategies have failed to demonstrate incremental reductions in death, stroke, worsening heart failure, or hospitalizations when compared with rate control.62–65 In the Pharmacologic Intervention in Atrial Fibrillation (PIAF) pilot study, rhythm control therapy did not produce superior quality of life or symptom improvements over rate control, and it came with a higher rate of hospital admissions and adverse drug effects.62
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree