ECMO and Ventricular Assist Devices
Andrew J. Lodge
Robert D. B. Jaquiss
INTRODUCTION
Mechanical circulatory support (MCS) in children is used for both profound respiratory and circulatory failure. Occasionally, these conditions coexist in the same patient. Extracorporeal membrane oxygenation (ECMO) has been relatively commonly used in neonates since the initial successful use in the mid-1970s. Indications include persistent pulmonary hypertension related to meconium aspiration, sepsis, idiopathic causes, and congenital diaphragmatic hernia. As results with this population improved, the use of ECMO was expanded to a variety of causes of respiratory and circulatory failure in other infants and children.
End-stage heart failure is a growing problem in the pediatric population in patients with primary cardiomyopathies and the increasing number who are surviving with repaired or palliated congenital heart defects. These patients are conventionally managed with a combination of oral and intravenous medications. When these fail, MCS is indicated. Particularly in small patients, ECMO has been historically used for this purpose. In adults, ventricular assist devices (VADs) have become the standard of care. There are a variety of devices that are approved by the U.S. Food and Drug Administration (FDA) for both short- and long-term support of these patients. Over the past several years, improvements in technology have now made VADs available to infants and children. These devices may be indicated as a bridge to recovery of the native heart function, or, more commonly in children, as a bridge to transplant. At the current time, there are devices in clinical use or being developed that will further improve the care and outcomes of these patients. This chapter will focus on the use of ECMO and VADs in children.
RESPIRATORY AND HEART FAILURE IN PEDIATRIC PATIENTS
Profound respiratory failure may occur in infants in children due to a variety of etiologies including those mentioned above as well as other infectious or inflammatory causes. In a minority of patients, conventional medical therapy including inhaled nitric oxide and alternative ventilation strategies may be inadequate to sustain adequate gas exchange. Severe respiratory failure in the neonate may precipitate pulmonary hypertension, shock, and circulatory collapse.
In contrast to adults, heart failure is relatively uncommon in pediatric patients. As in adults, the ultimate therapy for pediatric patients with end-stage heart failure is cardiac transplantation. Worldwide, 1,500 to 2,200 children are listed for heart transplant and each year 347 to 386 are transplanted. Congenital heart disease now surpasses primary cardiomyopathy as the most common indication for heart transplant in children. Since there are a growing number of children with successfully repaired or palliated congenital heart defects, one could anticipate that the number of listed patients and their waitlist times will increase. An important subset of this population is children with a diagnosis of hypoplastic left heart syndrome or related lesions. Results of conventional staged palliative surgery for these patients are improving, leading to a larger number of children with this diagnosis surviving. A number of these patients will experience failure of the single-ventricle circulation at various stages of palliation, including after the Fontan procedure. As a result, larger numbers of these patients will be evaluated for transplant. Compared with adults, the waitlist times for children are, on average, longer. Waitlist mortality is also higher in pediatric patients compared with adults with a threefold increase in the death rate on the waitlist that is essentially unchanged over the last decade. If medical therapy for advanced heart failure fails, some children will progress to the need for MCS. In the most recent era, the percentage of children supported with a VAD at the time of transplant was 13%, which has progressively increased over time.
RATIONALE AND INDICATIONS FOR THE USE OF EXTRACORPOREAL LIFE SUPPORT
Severe respiratory failure may become manifest by inadequate gas exchange despite maximal medical therapy, or excessive and toxic ventilator support being required to maintain such gas exchange. In such cases, ECMO is indicated to sustain life and allow time for lung recovery. Most recently, ECMO has been used successfully to bridge children with end-stage lung disease to lung transplantation.
End-stage heart failure may become manifest as refractory or unmanageable symptoms on conventional medications or, in babies, by poor growth, difficulty feeding, and failure to thrive. When more advanced, function of other organs such as the kidneys may become impaired. If these conditions do not improve with intravenous inotropes, MCS is indicated. In some children, end-stage heart failure is treated in part with tracheal intubation, sedation, and mechanical ventilation. This may reduce metabolic demand and oxygen consumption sufficiently to make the heart failure symptoms more manageable. However, remaining intubated and confined to bed has many risks. In adults, the use of VADs has allowed patients to improve from a metabolic, end-organ function, and nutritional standpoint. They can be extubated, become ambulatory, and even be discharged home as they await transplant. In many cases, this therapy has allowed patients who otherwise would have died to survive to transplant, and in others it has allowed them to become better transplant candidates. These same benefits can now be realized in children.
MCS may be indicated to treat postcardiotomy failure, acute cardiomyopathy, chronic cardiomyopathy, or failed corrective or palliative surgery for congenital heart disease. The indication, as well as
the intention to use MCS as a bridge to recovery, a bridge to transplant, or permanent (destination) therapy, has implications for the type of support chosen, the technical details of implementation, and the expected results and risks. Importantly, whenever the use of ECMO or VAD support is contemplated, careful thought should go into the eventual outcome. If a reversible cause of the underlying problem is unlikely, or if the patient is not a candidate for heart or lung transplant, MCS may be contrain-dicated.
the intention to use MCS as a bridge to recovery, a bridge to transplant, or permanent (destination) therapy, has implications for the type of support chosen, the technical details of implementation, and the expected results and risks. Importantly, whenever the use of ECMO or VAD support is contemplated, careful thought should go into the eventual outcome. If a reversible cause of the underlying problem is unlikely, or if the patient is not a candidate for heart or lung transplant, MCS may be contrain-dicated.
EXTRACORPOREAL MEMBRANE OXYGENATION
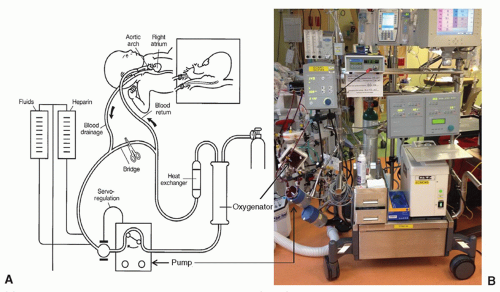
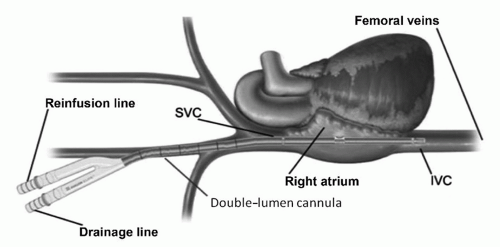
ECMO systems include a blood pump, an artificial lung (oxygenator), tubing, and monitoring equipment (Fig. 106.1). ECMO support is generally established by inserting relatively large-bore arterial and venous cannulae in peripheral vessels. ECMO may be venovenous (VV), in which case drainage and return of blood are both from the venous side of the circulation, or venoarterial (VA), in which case blood is withdrawn from the venous side and returned to the arterial circulation. VV ECMO is indicated for purely respiratory support and may be sufficient if cardiac function is relatively preserved and the patient is not in profound shock or on excessive inotropic or vasopressor support. In most cases, a specially designed double-lumen cannula, similar to a dialysis catheter, is used and placed through the right internal jugular vein to the right atrium (Fig. 106.2). An alternative is drainage from one cannula in the femoral vein and return via a different cannula in the internal jugular vein.
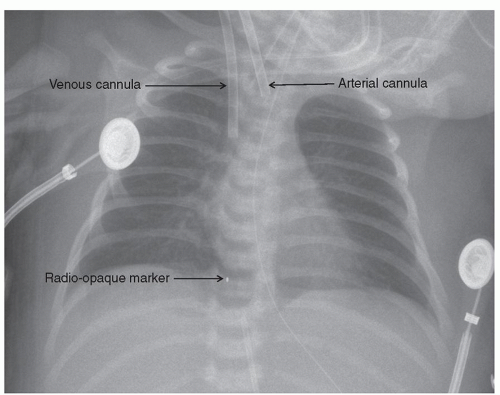
In infants and smaller children, the right common carotid artery and internal jugular vein have traditionally been used as these vessels are usually easily accessible and amenable to rapid exposure. These are typically accessed by a transverse skin incision on the right side of the neck approximately one to two fingerbreadths above the clavicle depending on the size of the child. The subcutaneous tissue and platysma are incised with the cautery. The medial border of the sternocleidomastoid muscle is mobilized. The right common carotid artery and internal jugular vein are isolated. Meticulous hemostasis is maintained since the patient will be anticoagulated. At this point, we administer 50 U/kg of intravenous heparin and allow 2 to 3 minutes for circulation. If VA ECMO is used, the carotid artery is cannulated first. Care is taken to avoid the vagus nerve, which is adjacent to the artery. A silk ligature is used to ligate the more cephalad portion of the exposed vessel. The ligature is clipped to the drape such that the vessel is placed on some traction. A loose ligature is placed around the artery proximally, and a fine vascular or bulldog clamp is used to temporarily occlude the vessel. Using a No. 11 blade, a transverse arteriotomy is made to open the artery. The appropriate-sized cannula, usually 8F or 10F for a neonate or small infant, is inserted to a depth of 2 to 3 cm and secured by tying the proximal ligature around the artery and cannula. We tie the ligature over a small piece of vessel loop to prevent damage to the vessel wall at decannulation. The more distal ligature is then tied around the cannula for security. The right internal jugular vein is cannulated in similar manner. Occasionally, a branch such as the facial vein requires ligation and division. The venous cannula, which has more side holes, is usually larger than the arterial by one or two sizes and is inserted to a greater depth such that the tip is well into the right atrium (Fig. 106.3). Both are then secured to the skin with two additional sutures each. The incision is then closed around the cannulae either with a simple running monofilament suture or with a layer of absorbable suture to reapproximate the platysma layer followed by the skin closure. Ideally, a small skin bridge
is left between the arterial and venous cannulae.
is left between the arterial and venous cannulae.
Depending on the center and clinical circumstances, when the child is weaned from ECMO support and the cannulae are removed, the vessels may remain permanently ligated or be repaired. It is unclear whether one approach is superior to the other. If the vessels are repaired, the ligatures must be removed carefully while maintaining proximal and distal control with a combination of vessel loops or fine vascular clamps. A small amount of back-bleeding is allowed to assess vessel patency and expel any debris or thrombus. The vessels are then repaired with fine, typically 7-0, monofilament sutures. Either a running or interrupted technique can be used.
In older children, there may be reluctance to ligate the carotid artery, although this has been done successfully in several centers. Other options for cannulation include the femoral, iliac, and axillary vessels. Like the cervical vessels, the femoral artery and vein are relatively superficial and amenable to reasonably rapid exposure by cutdown. Alternatively, if the child is large enough, with currently available cannulae, these vessels can be accessed percutaneously. The lower extremities are more susceptible to ischemic complications related to cannulation as collateral circulation is not as robust as in the brain. This can be treated with the placement of a more distal arterial catheter, which is connected to the arterial limb of the ECMO circuit. In small children, it may be difficult to get cannulae of adequate size to achieve full flow in the femoral vessels. In these cases, the external iliac vessels may be used. These vessels are accessed by performing an extraperitoneal dissection via an incision above the inguinal ligament. It is generally not safe to access these vessels percutaneously. If the axillary vessels are used, which is unusual, they can be accessed via an infraclavicular incision. The brachial plexus is at risk of injury from this exposure. Most commonly, this incision is used for arterial access only. A vascular graft is usually sewn end-to-side to the artery and the cannula is placed in the graft rather than cannulating the artery directly. The femoral, iliac, and axillary vessels are not permanently ligated after discontinuing ECMO.
PREPARATION FOR VENTRICULAR ASSIST DEVICE IMPLANTATION
The placement of any VAD requires meticulous preparation. The appropriate device should be selected based on the patient size and indication as discussed above. The patient should be free from any active infection. Neurologic status should be carefully assessed prior to surgery as an acute intracranial ischemic or hemorrhagic process represents a contraindication. The presence of any intracardiac shunting should be excluded by echocardiogram. If a shunt such as a PFO is present, it should be closed at the time of VAD implantation. The patient should be rendered as metabolically stable as possible, although not infrequently conditions such as metabolic acidosis and renal insufficiency represent part of the clinical syndrome that makes MCS necessary and will improve after successful VAD placement. Any preexisting coagulopathy should be reversed to the extent possible prior to surgery.
TEMPORARY VENTRICULAR ASSIST DEVICES
Although ECMO can be used for cardiovascular support and has some advantages such as the potential for rapid institution (which is useful in the setting of cardiopulmonary resuscitation) almost anywhere in the hospital and its combination of
cardiac and respiratory support, these are balanced against many negative features that make it suboptimal as a durable form of circulatory support. These include the relatively unstable cannulation, which has traditionally required the patient to be fairly immobilized, nonambulatory, and generally confined to a mechanical ventilation, the relatively large artificial blood contacting surface that results in hemolysis and platelet destruction, ongoing transfusion requirements and coagulopathy, and potentially inadequate decompression of the left ventricle leading to ongoing left atrial hypertension, pulmonary edema, and in the worst cases, pulmonary hemorrhage. In addition, inadequate unloading can impair any potential recovery of the compromised ventricle. In these situations, additional steps must be taken to decompress the left side of the circulation. This can be accomplished in the cardiac catheterization lab with atrial septostomy or septoplasty, or by the surgical placement of a left atrial or ventricular vent that is connected to the venous limb of the ECMO circuit. All of these factors make ECMO suitable for short-term support, but very suboptimal for longer term use.
cardiac and respiratory support, these are balanced against many negative features that make it suboptimal as a durable form of circulatory support. These include the relatively unstable cannulation, which has traditionally required the patient to be fairly immobilized, nonambulatory, and generally confined to a mechanical ventilation, the relatively large artificial blood contacting surface that results in hemolysis and platelet destruction, ongoing transfusion requirements and coagulopathy, and potentially inadequate decompression of the left ventricle leading to ongoing left atrial hypertension, pulmonary edema, and in the worst cases, pulmonary hemorrhage. In addition, inadequate unloading can impair any potential recovery of the compromised ventricle. In these situations, additional steps must be taken to decompress the left side of the circulation. This can be accomplished in the cardiac catheterization lab with atrial septostomy or septoplasty, or by the surgical placement of a left atrial or ventricular vent that is connected to the venous limb of the ECMO circuit. All of these factors make ECMO suitable for short-term support, but very suboptimal for longer term use.
Better temporary support options may include centrifugal pump-based VAD systems. These systems, which employ a centrifugal blood pump, tubing, and cannulae similar to those used for cardiopulmonary bypass (CPB), are designed for short-term use, can be customized to provide single or biventricular support, can be adapted to fit a variety of different sized patients, and are relatively inexpensive. Temporary cannulation is employed, which is not typically stable enough to allow much patient movement. Frequently, these types of systems are placed with only temporary sternal closure in the postcardiotomy support setting, which limits the ability to mobilize the patient. Until recently, pediatric-specific pumps were not available, leading to potential problems with lower pump speeds predisposing to thrombosis and thromboembolism in this group. Recently, the Levotronix® Corporation (Waltham, MA) developed a smaller version of their magnetically levitated centrifugal blood pump, the CentriMag®, called the PediMag® that obtained 510k clearance from the FDA in 2009 for short-term use as a pediatric blood pump (Fig. 106.4). This pump has a priming volume of only 14 ml and 1/4 inch connections, and a maximum flow of 1.5 l/min making it potentially useful as a temporary VAD for infants and small children. The PediMag® has been successfully used as a bridge to transplant in an infant. Its use may increase as further experience with it is gained.
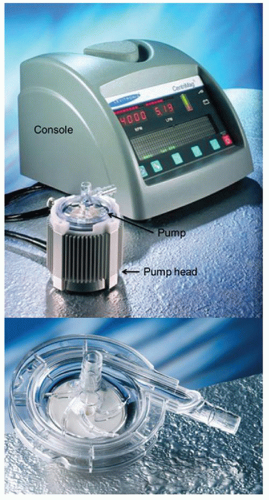 Fig. 106.4. A CentriMag® console and pump and PediMag® pump are shown. The PediMag® is similar in design to the CentriMag and uses the same console but is smaller and has ¼ inch connections. |
Cannulation for LVAD support is usually accomplished with direct aortic cannulation for outflow and cannulation of either the left atrium or the left ventricular (LV) apex for inflow. Apical cannulation provides more complete ventricular decompression, whereas atrial cannulation is more easily accomplished but may risk ventricular thrombus formation due to stagnant flow. RVAD support is generally accomplished by cannulation of the right atrium for inflow and the pulmonary artery for outflow. All cannulae should be secured with two concentric purse-string sutures, which are securely tied to the cannulae themselves to maximize hemostasis and immobilization. Cannulae may be selected that can be tunneled through the body wall like a chest tube to permit chest closure during the period of support. When these devices are used for postcardiotomy support with anticipated ventricular recovery, weaning at the bedside can be accomplished reasonably easily, and the temporary nature of the cannula placement facilitates device removal if sufficient recovery occurs.
PARACORPOREAL DEVICES
The most commonly used VADs in children today are so-called first-generation paracorporeal devices. These are displacement pumps that function much like the native ventricle with inflow and outflow valves and a pumping chamber that is pneumatically driven. These devices can be used for single or biventricular support, but employ cannulation that is stable and allows for patient extubation, mobilization, and rehabilitation. In older children, adult devices such as the Thoratec paracorporeal VAD (PVAD; Thoratec Corporation, Pleasanton, CA) have been used. Unfortunately, the blood pump and cannulae for the device are large, which leads to potential problems with thromboembolism, excessive stroke volume, low pump flow rates, and difficulty with cannula positioning. From a practical standpoint, these systems cannot be used in children under 20 kg, and even in that size range, the use of the pump is problematic.
The most important current manufacturer involved in pediatric VAD development is Berlin Heart GmbH (Berlin, Germany). In 1989, they modified an adult device in order to allow VAD placement in small children (Fig. 106.5). The Berlin Heart EXCOR® Pediatric VAD is produced in a variety of sizes, with an applicable size range from 3 kg to adult size. For approximately a decade, the use of the device was confined to Western Europe. After the first usage of the device in the United States was reported in 2005, its use spread to multiple centers in North America, all under so-called compassionate use protocols. The positive experience at implanting centers led to the initiation in 2007 of a large multicenter trial, constructed to potentially permit formal FDA approval of the device. This trial has now been completed and has resulted in the recent approval of the device in the United States.
Implantation of all the paracorporeal devices is similar. For LVAD support, apical cannulation for device inflow is preferred as discussed above. Using normothermic or mildly hypothermic CPB, the pericardium is opened and the apex of the heart is identified. A skin exit site is selected for the inflow cannula in the left upper quadrant below the costal margin on a line corresponding to the location of the apex. A disc of skin slightly smaller than the diameter of




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree