BACKGROUND
The clinical picture of infective endocarditis continues to evolve with changes in the populations at risk, development of new diagnostic tools, improvements in surgical management, and evolution of antibiotic susceptibilities of organisms. The frequency of endocarditis in children appears to be increasing with the current incidence estimated at 0.8 to 3.3 per 1000 pediatric hospitalizations. This increase is often attributed to three major sources: (a) the growing population of individuals with repaired or palliated congenital heart disease, (b) the explosion in the use of indwelling intravenous lines in both neonatal and pediatric intensive care units, and (c) an increase in the use of intracardiac devices and prostheses. A bimodal age distribution for children with endocarditis has been reported, with peaks in infancy (3–11 months) and late teenage years (17–20 years). Although mortality has decreased from 100% in the preantibiotic era to the current rate of 5% to 30% at most pediatric institutions, endocarditis remains a severe disease often accompanied by a high morbidity. At best, endocarditis in children requires prolonged hospitalization and usually 4 to 6 weeks of intravenous antibiotics.
RISK FACTORS
Endocarditis may occur in anyone. However, populations at risk have been defined (Table 30.1), and the index of suspicion should be high in these cases. This is particularly true in the child who has multiple risk factors such as the premature infant with underlying congenital heart disease, an immature immune system, and an indwelling intravenous line for parenteral nutrition. Rheumatic fever, now rare except in specific areas of the United States, has been replaced by indwelling lines, prosthetic valves, intracardiac devices, and congenital heart disease as the highest risk factors for endocarditis in developed countries.
PATHOGENESIS
Bacteria that commonly lead to endocarditis include viridans streptococci, Staphylococcus aureus and S. epidermidis, beta-hemolytic streptococci, and some gram-negative organisms. Polymicrobial endocarditis is uncommon in children, but when it does occur, it is largely limited to intravenous drug users. The most common fungal etiologies of endocarditis are Candida and Aspergillus. Regardless of the organism involved, the pathogenesis is similar. Bacteremia frequently occurs in all individuals in many settings, including postoperatively, with intravenous drug use, or with daily activities such as chewing, tooth brushing, or flossing. Although bacteremia occurs frequently, it rarely leads to endocarditis. Endocarditis only occurs when virulence factors and a unique cytokine milieu allow bacteria to adhere to exposed fibronectin. For example, turbulent blood flow from congenital or acquired heart disease, especially in lesions with high pressure gradients, may traumatize and disrupt endothelium, exposing fibronectin and initiating the clotting cascade and fibrin deposition that leads to clot formation (nonbacterial thrombotic endocarditis). In the setting of bacteremia, bacteria can invade the clot and vegetation growth encases the original nidus of infection. As the infection progresses, it destroys surrounding tissue. The location and amount of tissue destroyed determine the degree of valve regurgitation as well as the presence and size of an abscess cavity.
CLINICAL PRESENTATION
The clinical presentation of the child with endocarditis depends on age, general state of health, and causative organism. Presentations vary widely within age groups and with the causative organism, so the terms “acute” and “subacute” are no longer widely used. Premature or sick neonates requiring central indwelling lines for parenteral nutrition (Fig. 30.1, Video 30.1) are at risk regardless of the presence or absence of a cardiac anomaly. Coagulase-negative staphylococci and enterococci are important pathogens in this age group. The sick neonate may or may not have fever, and other systemic signs may be nonspecific, making the diagnosis of endocarditis difficult. Therefore, the standard of care at many institutions includes a complete echocardiogram in all neonates with indwelling lines who have recurrent or persistent bacteremia to look for vegetations and determine the need for line removal.
The clinical presentation in older children is also variable. Although Osler’s triad of pneumonia, endocarditis, and meningitis is considered the classic finding in adults, it rarely occurs in children. In children, the disease is often indolent and characterized by fever, weight loss, fatigue, and night sweats. Myalgia, arthralgia, headache, and general malaise are common. Nearly all children with endocarditis have a murmur. Some investigators report an average of 35 to 90 days between the first symptom and diagnosis and suggest that the nonspecific and insidious development of symptoms may delay the diagnosis. Viridans group streptococci remain the most frequent cause of endocarditis in children. It commonly presents with an indolent course and rarely leads to complications, especially if diagnosed early and treated with proper antibiotic therapy. Because endocarditis can result in generalized, nonspecific symptoms in children with normal hearts or those with minor unrecognized congenital heart disease, it should be considered in the differential for all children with prolonged, unexplained fever with persistent bacteremia.
Severe infection with valve destruction and significant regurgitation leads to symptoms of congestive heart failure and a severely ill child. In this scenario, a skilled clinician with a high index of suspicion readily makes the diagnosis. The challenge in evaluating a febrile child with less involvement, however, lies in differentiating the innocent murmur associated with the high-output state often associated with fever from the murmur of valve regurgitation. When in doubt, echocardiography should readily resolve the question as to the etiology of the murmur. However, it should be noted that the yield for a positive study indicative of endocarditis is low in the absence of positive blood cultures and appropriate clinical setting.
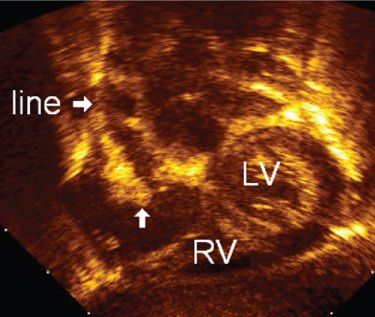
FIGURE 30.1. Modified subcostal view of a central venous line in the right atrium. The use of B-color allows easy identification of the dense vegetation (arrow), giving a thickened, irregular appearance to the catheter. LV, left ventricle; RV, right ventricle.
As stated above, bacteremia is common with daily activities but rarely results in endocarditis. The exception to this rule is when S. aureus is a causative organism. S. aureus causes many pyogenic infections in children and may lead to sepsis. Endocarditis develops in about 20% of cases of staphylococcal sepsis, even in the absence of underlying heart disease. Because the classic findings of endocarditis are seldom present and the clinical picture is often nonspecific, many experts recommend the routine use of echocardiography with all cases of S. aureus sepsis.
Circulating immune complexes and rheumatoid factor occur in 84% to 100% of cases of endocarditis and are deposited in the skin, spleen, synovium of joints, and glomerular basement membrane. These cause the classic noncardiac manifestations associated with endocarditis, including splenomegaly, polyarthritis, glomerulonephritis, splinter hemorrhages, Osler nodes (painful bluish nodules at the fingertips), Janeway lesions (painless hemorrhagic lesions on the palms and soles), and Roth spots (retinal hemorrhages).
Complications of endocarditis may occur early and alter the presentation as described in the next section.
COMPLICATIONS
Complications (Table 30.2) from endocarditis are common and contribute to the morbidity and mortality. Risk factors for complications include prosthetic valves, S. aureus or fungal causative agents, prior episodes of endocarditis, cyanotic heart disease, left-sided involvement, and clinical symptoms for longer than 3 months.
Congestive heart failure is one of the most common complications of endocarditis. Heart failure may occur acutely and abruptly as a result of valvular regurgitation occurring when native valve leaflets or support structures are disrupted (Fig. 30.2) or from dehiscence of a valve prosthesis resulting in a significant paravalvar leak. In other cases, heart failure symptoms may develop more slowly or insidiously. In either case (acute or insidious), heart failure is secondary to valve regurgitation with or without accompanying ventricular dysfunction. Surgical intervention should be considered in patients with endocarditis and heart failure.
Embolization of vegetation fragments complicates endocarditis in 20% to 40% of cases. The risk is highest during the first days after antibiotic therapy is initiated and drops to 9% to 21% after 2 weeks of treatment. Some studies report the most common noncardiac complications involve the brain, occurring in up to 20% of patients. Stroke preceded the diagnosis of infective endocarditis in up to 60% of patients with endocarditis complicated by stroke. The lungs, kidney, and spleen are also commonly affected. Coronary artery embolism is a less common but potentially lethal complication.
Embolism occurs frequently in gram-negative, pneumococcal, or fungal endocarditis. In children, gram-negative endocarditis is most commonly caused by one of the HACEK organisms (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella spp.) and is associated with embolism in 31% of cases. About one-third of cases of HACEK endocarditis occur in the absence of underlying heart disease. Because these organisms are fastidious and slow growing and require special growth factors and carbon dioxide for isolation, diagnosis and appropriate treatment are typically delayed, allowing the vegetations to become large and friable, increasing the risk of embolization.
There has been a resurgence of pneumococcal endocarditis in parallel with the increase in its antibiotic resistance. Pneumococcal endocarditis is associated with meningitis, pneumonia, otitis media, or mastoiditis in 50% of pediatric cases. The mitral valve is involved (either alone or in association with aortic or tricuspid valve) in 68% of cases, and 80% have large vegetations, predisposed to embolization.
Fungal organisms account for approximately 1.1% of cases of endocarditis in children, occurring most commonly in developing countries where it is often fatal. Candida causes about 63%, and Aspergillus, 26% of cases. Approximately two-thirds of cases involve children younger than 1 year. In developed nations, premature neonates undergoing medical instrumentation and children who have had congenital heart surgery using prosthetic material are at highest risk of developing fungal endocarditis. Diagnosis is often delayed until large, friable vegetations have formed or after embolization has occurred. The major obstacle to diagnosis is the difficulty in reliably growing fungi in automated blood culture systems. Polymerase chain reaction (PCR) has been useful for the detection and identification of fungal organisms.
One of the most severe complications of endocarditis is an abscess. A paravalvular abscess may be seen when the infection extends beyond the valve annulus. Although this is a rare complication, it is strongly associated with a poor outcome. In both children and adults, S. aureus is the most common cause and nearly all abscesses involve the aortic valve. In adults, the incidence of paravalvular abscess is approximately 20% to 40% with native aortic valve endocarditis and 60% in the setting of a prosthetic aortic valve. Similar data are not available for children as only small series or case reports comprise the literature. The literature suggests that the underlying native valve anatomy is normal in 66% of cases of endocarditis complicated by abscess formation in children. Although a paravalvular abscess may have an indolent presentation, the more typical picture is that of acute, severe illness. The child has a new pathologic murmur and may also have a rub indicating pericarditis. The abscess disrupts the aortic annulus, creating severe regurgitation that results in intractable congestive heart failure (Fig. 30.3). The infection may extend into the mitral-aortic fibrosa and involve the anterior mitral leaflet so that the mitral valve becomes regurgitant as well. When the structural integrity of the aortic wall is further weakened, a sinus of Valsalva aneurysm may form and ultimately rupture into the right ventricular outflow tract or either atria, creating a shunt that is poorly tolerated. Fever and bacteremia usually persist despite appropriate antibiotic therapy and recurrent emboli are common. The new development of bundle branch block or complete heart block is highly specific for a paravalvular abscess. Most experts recommend serial echocardiographic evaluations looking for development of paravalvular abscess in the following settings: (a) S. aureus endocarditis regardless of whether a native or prosthetic aortic valve is involved, (b) persistent fever or bacteremia despite appropriate antibiotic therapy, (c) a persistent bundle branch block or complete heart block, especially when there has been a prolonged period of symptoms before diagnosis and treatment, and (d) thickening of the aortic root is visualized by echocardiography in a patient with endocarditis.
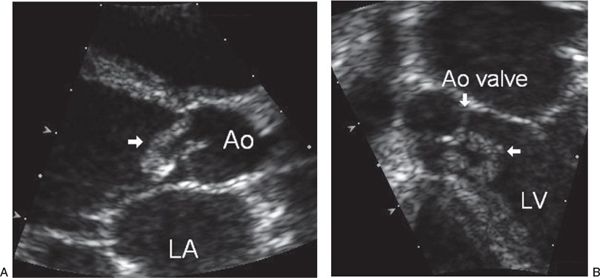
FIGURE 30.2. Aortic valve vegetation. A: Parasternal long-axis view shows a vegetation on the aortic valve (arrow) that prolapses into the left ventricle during diastole. The valve cusps are partially destroyed and prolapsed, resulting in severe aortic regurgitation. Ao, aorta; LA, left atrial. B: Apical four-chamber view demonstrates the extensive involvement of the vegetation (horizontal arrow) on the aortic valve (vertical arrow) that extends along the interventricular septum. Ao, aorta; LV, left ventricle.
DIAGNOSIS
Endocarditis is largely diagnosed by positive blood cultures for a typical organism and the presence of vegetations and/or abscess formation on echocardiography. Systemic, vascular, and/or immunologic findings support the diagnosis. Typical laboratory findings include anemia and elevated white blood count, erythrocyte sedimentation rate, and C-reactive protein. Blood cultures are positive in over 90% of cases but may be negative with prior use of antibiotics, with right-sided endocarditis, or when the pathogen is intracellular or fastidious. Serology may prove useful in these circumstances. Serology is the only method that allows diagnosis of Coxiella burnetii (Q fever), Brucella, Bartonella, and chlamydial endocarditis.
Early diagnosis and aggressive treatment are warranted to avoid the serious complications associated with endocarditis. Although infective endocarditis has been diagnosed using bedside, focused ultrasound in the emergency department in isolated instances, more data are needed before this modality can be recommended as an effective and accurate approach to the diagnosis of endocarditis. It is also important to avoid overdiagnosis, as treatment involves weeks or months of intravenous antimicrobial therapy. When the classic noncardiac findings and/or new-onset regurgitant murmurs are present in the febrile child with bacteremia and an echocardiogram shows vegetations and/or abscess, the diagnosis of endocarditis can readily be made. The picture is seldom so clear, however. Fever may be absent if the child is debilitated, immunocompromised, or pretreated with antibiotics. Innocent murmurs are common in children during illness and may be difficult to differentiate from those of valvar regurgitation. Noncardiac manifestations may not be present. In a series of 76 episodes of endocarditis in children, splenomegaly occurred in 5%, splinter hemorrhages in 4%, Roth spots in 4%, and Osler nodes in 3%.
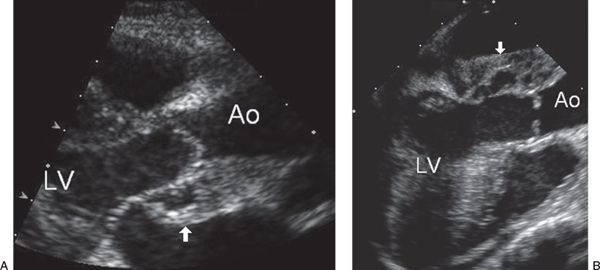
FIGURE 30.3. Paravalvular abscess. A: Parasternal long-axis view of the left ventricular outflow tract shows the shaggy appearance of a hypoechoic space posterior to the aortic outflow tract representing a paravalvular abscess (arrow). Ao, aorta; LV, left ventricle. B: Transesophageal echocardiogram using a longitudinal view of the left ventricular outflow tract in the same patient demonstrates the abscess cavity (arrow). The aortic valve leaflets are thickened and distorted and fail to coapt with significant aortic regurgitation. Ao, aorta; LV, left ventricle.
A team of primary care physicians, infectious disease specialists, cardiologists, and cardiovascular surgeons may be needed for optimal evaluation and management. The modified Duke criteria (Table 30.3) have been validated in children and should be used when the clinical picture is unclear. The Duke criteria function similar to the Jones criteria for rheumatic fever by dividing clinical, microbiological, and echocardiographic findings into major and minor categories. The two major criteria typically include (a) microbiologic evidence and (b) evidence of endocardial involvement (positive echocardiogram or new valvular regurgitation). It is worth noting that three echocardiographic findings qualify as major criteria: (a) discrete, echogenic, oscillating intracardiac mass at the site of endocardial injury, (b) paravalvular abscess, or (c) new dehiscence of a prosthetic valve. The 15 minor criteria include the presence of specific known risk factors, evidence of emboli, classic findings resulting from immunologic phenomena, and laboratory findings. Endocarditis is definitively diagnosed if (a) there is pathologic evidence of an intracardiac or embolized vegetation or intracardiac abscess or (b) fulfillment of clinical criteria, requiring the presence of (i) two major or (ii) one major and three minor or (iii) five minor criteria. Endocarditis is considered possible if one major and one minor or three minor criteria are present. A firm alternative diagnosis, resolution of symptoms within 4 days of antimicrobial therapy, and absence of surgical or autopsy evidence of endocarditis within 4 days of antimicrobial therapy points away from a diagnosis of endocarditis.
Blood cultures are central to the diagnosis and effective treatment of endocarditis. Contrary to what is considered optimal in other settings, it is not necessary to obtain blood sampling at the time of fever, as the bacteremia of endocarditis is continuous. The volume of blood drawn is important, however, and experts recommend 1 to 3 mL in infants and 5 to 7 mL in older children. Three cultures over a minimum time of 1 hour should be drawn before administering empiric antibiotics. Despite numerous cultures with adequate volumes, prolonged culture periods, and examination of surgically excised tissue, about 5–10% of cases of endocarditis are culture-negative. Attempts to obtain an organism through antibody titers and polymerase chain reaction techniques may also fail. Reasons for “culture-negative” endocarditis include inability to grow a fastidious organism, antibiotic use prior to culturing, pulmonary filtering of bacteria for right-sided lesions, and sequestration of the organism within the vegetation.
MANAGEMENT
It is important to note that the Duke criteria provide guidelines for the diagnosis but not the management of endocarditis. Administration of intravenous antibiotics is clearly indicated for those with definite endocarditis, but their use for possible or rejected cases must be considered on an individual basis. The team of experts must weigh the risk factors, evaluate the reliability of the blood cultures, and determine the likelihood that endocarditis best explains the clinical picture.
Once diagnosed, the patient’s condition and causative organism dictate the course and length of treatment. All patients require medical therapy and some will require surgery. If antibiotics are started before culture results become available, treatment is usually directed toward the most common bacteria—streptococci and staphylococci. Once the organism is isolated, susceptibilities are determined and antibiotics adjusted accordingly. Antibiotics may be combined to provide synergy and must be given intravenously to achieve concentrations sufficiently high to treat infection in the poorly vascularized valve leaflets as well as penetrate infected vegetations. Treatment is typically for 4 to 6 weeks but may be longer in those patients with prosthetic valves or those who are immunocompromised or have other extenuating circumstances. Anticoagulation is not indicated (unless given for a reason other than endocarditis) and is actually contraindicated with severe cerebral complications or mycotic aneurysms. Afterload reduction in the setting of significant acute aortic or mitral regurgitation is limited to stabilization of the patient before surgical intervention.
While surgery is indicated in patients with cardiogenic shock or life-threatening congestive heart failure due to valve disease from endocarditis, the indications for surgical intervention in the hemodynamically stable patient are not as clear. Those indications with the strongest evidence include abscess formation, severe valvular regurgitation with intractable congestive heart failure, recurrent arterial emboli, fungal infection, and persistent bacteremia after 1 week of appropriate antibiotic therapy (Table 30.4).Typically, patients with infected pacing systems (Fig. 30.4, Video 30.2), intracardiac devices (Fig. 30.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


