INTRODUCTION
Background
Since the introduction of the first ventricular assist devices (VADs) and extracorporeal membrane oxygenation (ECMO) in the 1950s–1960s, mechanical circulatory support (MCS) has become a mainstay of treatment for patients with acute, life-threatening heart failure. Initially used in the adult population, MCS utilization continues to increase in infants and children. Consequently, echocardiographic assessment of the heart on MCS has become more common, particularly in the intensive care unit (ICU).
MCS systems have been shown to be useful for supporting hemodynamics in patients with acute, recoverable heart failure such as acute fulminant myocarditis, as well as in those with acute cardiac dysfunction following congenital heart surgery. Additionally, MCS has become an effective “bridge” to heart, lung, or heart-lung transplantation, supporting the patient with cardiopulmonary dysfunction while awaiting donor organ availability. Recently, some centers have utilized a “bridge to decision” strategy, where MCS has been used to delay decisions about transplantation until further testing or procedures can take place. Other indications for MCS include support for patients with refractory arrhythmias, for heart failure patients who need to initiate pharmaceutical regimens, and for patients with life-threatening cardiac defects that cannot undergo immediate surgical repair.
The different types of MCS currently available for children are listed in Table 37.1. MCS systems can effectively be differentiated by their expected duration of support in a particular patient. Short-term devices may offer support for days or even up to a few weeks, and include ECMO, intra-aortic balloon pumps, and centrifugal ventricular assist devices. Long-term devices may offer support for a few weeks up to years, and include most paracorporeal and implantable VADs. Selection of a particular device for any given patient is dependent on the specific patient anatomy, age and size, and expected duration of support. The specific device chosen may also be highly institution-dependent, with factors including surgeon and cardiologist preference, experience with a particular device, and the input of collaborating adult teams when available/applicable.
Importance of Echocardiography
Echocardiography plays a critical role in the evaluation of the patient with end-stage or acute heart failure, including diagnostic and functional assessments. In addition, it is the most frequently utilized imaging technique prior to, during, and after placement of the patient on MCS. Finally, echocardiography can be useful in the determination of myocardial recovery in selected patients. In the following chapter, we will discuss echocardiographic assessment of the patient before, during, and after placement on MCS, with a focus on VADs. This will be followed by a discussion of the specific roles of echocardiography in other types of MCS, including ECMO and percutaneous devices.
ECHO ASSESSMENT BEFORE MCS PLACEMENT
Diagnosis
Echocardiography plays a central role in the evaluation and management of patients for whom MCS is being considered. First and foremost, echocardiography can establish the putative diagnosis and assist in the determination of the type of device (short- versus long-term support, implantable versus paracorporeal). Echocardiography allows for detailed assessment of systolic and diastolic function of the ventricles (left, right, or single), and can help the clinician in the determination of the timing of MCS initiation. Importantly, echocardiography allows for evaluation of the structural anatomy of the heart and for the presence of any residual cardiac lesions. This assessment is critical for deciding the location of insertion of both inflow and outflow cannulae. If the patient has a history of corrective or palliative congenital heart surgery, the specific connections that have been made need to be taken into account when considering MCS choice and location. For all of these reasons, it is critical to have echocardiographers and surgeons trained in congenital heart disease making these pre-MCS assessments in children.
In cases of VAD placement in a biventricular heart, echocardiography can help the clinician decide whether a systemic VAD (most often supporting the left ventricle, LVAD) alone will be sufficient for the patient, or whether biventricular support (addition of RVAD to support the right ventricle) will be required. Primarily, this decision involves evaluation of the subpulmonary ventricle (most often the right ventricle) to determine function, size, and valvar function. A poorly functioning right ventricle may benefit from the reduction in afterload that occurs with an LVAD. However, the right ventricle may not be able to adapt to the increased preload from increased systemic output. Studies in adults have suggested that severely enlarged right ventricles (end-diastolic volume >200 mL) have a higher propensity for failing when the patient is placed on an LVAD.
Valve Function
Valve function can be assessed comprehensively using echocardiography. Significant valvar regurgitation or stenosis can have a deleterious effect on MCS capability, sometimes requiring modification of the technique.
Aortic insufficiency is very important to assess prior to VAD placement. Not uncommonly, the degree of aortic insufficiency may appear to worsen after placement of a VAD. This is likely due to underestimation of the severity of regurgitation in the setting of severe ventricular dysfunction. When severe, aortic insufficiency causes the VAD circuit to be inefficient, with much of the VAD output lost back into the ventricle. If this occurs, aortic valve replacement or oversewing the valve should be considered at the time of VAD implantation. Aortic stenosis, when present, is often not a major concern in patients with pulsatile VADs; however, it becomes an issue for any device with partial support, or those which cross the aortic valve (such as the Impella Recover, Abiomed, Danvers, MA).
Mitral valve dysfunction commonly occurs in patients with severely dilated left ventricles in the setting of dilated cardiomyopathy. This can result from annular dilatation as well as from ischemia of the papillary muscles. Most often, appropriate decompression of the left ventricle after VAD initiation will result in improvement in this functional mitral valve regurgitation. Mitral stenosis may affect inflow to an LVAD apical cannula. In this situation, the inflow cannula may need to be placed in the left atrium, or the mitral valve might require surgical intervention.
In patients with poor right ventricular function, significant tricuspid regurgitation may be an important association and it often results in worsening of cardiac output. Tricuspid regurgitation may be exacerbated by the septal shift that occurs with decompression of the left ventricle. Consideration should be given for addressing clinically significant degrees of tricuspid regurgitation at the time of VAD implantation (i.e., moderate or greater). Alternatively, the tricuspid regurgitation may be indicative of poor right ventricular function, and the clinician should be prepared for the possibility of needing biventricular support (BiVAD).
Pulmonary valve problems including insufficiency and stenosis are rare in patients undergoing VAD placement. In the setting of RVAD (or biventricular VAD), severe pulmonary insufficiency may result in inefficiency of the RVAD circuit, with much of the outflow lost back into the ventricle. Similar to patients with severe aortic insufficiency, patients with significant pulmonary insufficiency may rarely require valve repair or oversewing techniques.
Shunts
Detection of intracardiac shunts is important in the patient being placed on MCS, and is particularly critical in the VAD patient. Defects of the atrial septum allow for atrial level shunting. This is of particular importance in patients with poor biventricular function being supported by an LVAD alone. Right-to-left atrial shunting occurs due to decompression of the left atrium and ventricle after LVAD placement. Such shunting may result in hypoxia or paradoxical embolism. Thus, intracardiac shunts should be closed during VAD placement. In a study of 32 pediatric patients being placed on a Berlin Heart EXCOR device (Berlin Heart AG, Berlin, Germany), 11 were found to have intracardiac shunts prior to placement of the device. Surgical closure was performed in all.
Thrombus Formation
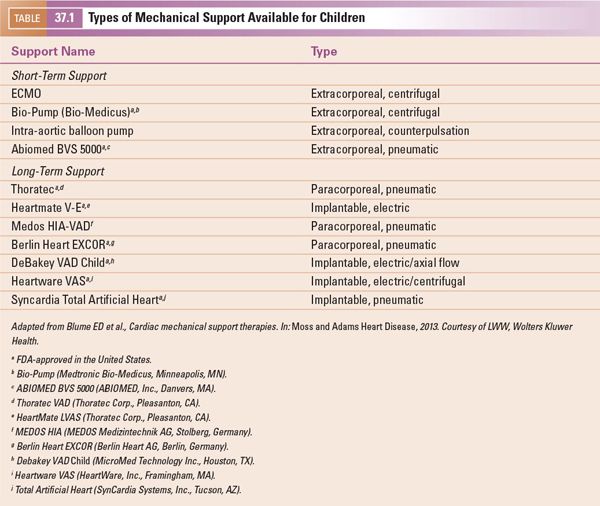
Assessment of the heart for thrombus formation is important, and may affect where the surgeon is able to place the inflow cannula. Common locations for thrombus formation include the systemic ventricular apex (the most common insertion site for an inflow cannula (Fig. 37.1, Video 37.1A,B)), and the atrial appendages. The ascending aorta, a common location of insertion of the outflow cannula, may harbor atheromatous disease in adults, but this is rarely a concern in children.
Figure 37.1. Magnified apical four-chamber view with focus on the left ventricle in a 2-year-old patient with dilated cardiomyopathy and poor systolic function. Note the thrombus in the left ventricular apex measuring 6 × 8 mm.
INTRAOPERATIVE ASSESSMENT OF MCS
Cannula Position
Transesophageal echocardiography (TEE) can be very effective in guiding placement of the cannulae during VAD implantation. The inflow cannula in the apex of the heart can be guided away from the ventricular walls, avoiding potential sources of obstruction (Fig. 37.2). In a typical four-chamber imaging plane, the inflow cannula should align with the mitral inflow stream. Color Doppler should demonstrate unidirectional, laminar flow into the inflow cannula (Fig. 37.3, Video 37.2A,B). Appropriate decompression of the left ventricle can be assessed (Fig. 37.4, Videos 37.3 and 37.4A-C). Similarly, outflow cannula placement can be guided in the aorta or pulmonary artery (Fig. 37.5, Videos 37.5 and 37.6). The outflow cannula in an LVAD is best visualized in the long-axis view of the aorta, typically from the mid-esophagus (~120 degrees). Doppler velocities up to 2 m/s are often seen on interrogation of the outflow color Doppler signal.
When an RVAD is placed, similar views can be obtained of the inflow and outflow cannulae. The inflow cannula in the right atrium can often be best visualized in a bicaval view (~120 degrees looking towards the atrial septum). The outflow cannula in the pulmonary artery is quite anterior and is therefore more difficult to visualize using TEE. However, with sufficient image quality, a short-axis view at the base of the great vessels may allow for visualization of the outflow cannula.
De-airing
In every VAD placement, a thorough evaluation for intracardiac air is mandatory prior to unclamping the aorta and taking the patient off of cardiopulmonary bypass. Intracardiac air can be detected as small bubbles noted on TEE imaging. Common entrapment sites for air bubbles include the anastomotic sites of the great vessels, as well as the anterior portions of the left and right ventricles, the left atrium, and the left atrial appendage.
Figure 37.2. A:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


