Ebstein’s Malformation of the Tricuspid Valve in Children
Sitaram M. Emani
Pedro J. del Nido
Ebstein’s malformation refers to a specific congenital defect of the tricuspid valve (TV) in which displacement of the hinge points of the septal and posterior leaflets toward the apex results in “atrialized” portion of the right ventricle (RV) and varying degrees of tricuspid regurgitation. The functional RV beyond the tips of the abnormal leaflets may be small, thin-walled, and hypokinetic or dyskinetic. The preoperative assessment, timing of intervention, and techniques for TV repair are discussed. Although an “ebsteinoid tricuspid valve” may also accompany L-transposition of the great arteries, the management of this entity is not discussed in further detail.
PREOPERATIVE PLANNING
Severity of Leaflet Abnormality
Ebstein’s malformation can be categorized according to the severity of apical displacement and hypoplasia of septal and anterior leaflets, as well as by the size of atrialized chamber. Echocardiography and magnetic resonance imaging (MRI) are typically used to categorize the malformation into types A-D, with type A describing very mild displacement of the septal leaflet, a large “saillike” anterior leaflet, and a small atrialized chamber. Type D describes near absence of functional tricuspid leaflet tissue and severe apical displacement of hinge points of both anterior and septal leaflets, resulting in a massive atrialized chamber, and orientation of the inflow orifice toward the RV infundibulum. The categorization is useful in surgical planning, as patients with more severe forms of the malformation are more likely to require leaflet substitution with prosthetic material and RV unloading with cavopulmonary shunting.
Atrial Septal Defect
In patients with Ebstein’s malformation and atrial septal defect, the direction of flow across the defect indicates relative compliance of the downstream ventricles. Gradual worsening of cyanosis heralds the decline in RV compliance and function. Catheter-based closure of the atrial septal defect in these patients may alleviate cyanosis but does not improve long-term prognosis related to ongoing RV volume overload and progressive RV failure.
Pulmonary Valve Stenosis or Atresia
Fetal or postnatal echocardiogram may demonstrate pulmonary atresia, but imaging may not reliably distinguish functional versus anatomic atresia in a fetus or infant with a patent ductus arteriosus (PDA) and significant left to right flow. The presence of pulmonary regurgitation suggests functional rather than anatomic atresia. The distinction is clinically relevant since the patient with anatomic atresia is dependent upon the PDA for pulmonary blood flow, whereas ductal closure may be the desired treatment for a neonate with functional atresia since interruption of the PDA restores antegrade flow across the pulmonary valve. Both functional and anatomic atresia are risk factors for mortality in neonates with Ebstein’s malformation. Similarly, RV outflow tract obstruction is a risk factor for poor outcomes in non-neonates undergoing repair.
Right Ventricular Function and Mass
MRI can be used to estimate the volume and wall thickness of the functional RV in the evaluation of Ebstein’s malformation beyond infancy. A dilated and thin-walled RV may not tolerate isolated TV repair, and unloading maneuvers, such as cavopulmonary shunting, may be necessary adjuncts. In the evaluation of the neonate or fetus, Doppler estimation of the RV pressures allows risk stratification. Although low-RV pressure is almost universally present in children and adults with Ebstein’s malformation, estimated RV pressures by echocardiography of <20 mmHg may be a sign of inadequate RV function in neonates.
Inducible Atrial and Ventricular Arrhythmias
Preoperative electrophysiologic studies are helpful at identifying the presence of accessory pathways or substrate for atrial or ventricular arrhythmias. Although ablation of accessory pathway can be performed in the catheterization laboratory, the presence of inducible atrial arrhythmias is an indication for intraoperative cryoablation-MAZE in a patient undergoing TV repair. The presence of inducible ventricular arrhythmias should raise consideration for intraoperative epicardial defibrillator coil placement, thus avoiding subsequent need for transvenous lead placement across the TV.
SURGICAL INDICATIONS AND TIMING
Operative intervention for Ebstein’s malformation typically follows a bimodal distribution, with initial phase occurring in neonates and infants, followed by a phase that occurs in adolescence and adulthood. The indications for operation, the type of procedure performed, and the operative mortality vary significantly for these two groups.
Neonates
Prenatal diagnosis of Ebstein’s malformation has allowed improvements in immediate postnatal management of this disease. Risk stratification prenatally can be performed by the assessment of RV size and function as well as by the measurement of RV pressures and direction and degree of flow across the PDA and right ventricular outflow tract. In severe forms of Ebstein’s malformation, the RV pressure estimated by Doppler velocity of tricuspid regurgitation jet is low (<20 mmHg), suggesting poor systolic function of the RV. Presence of anatomic pulmonary atresia is a risk factor for mortality in neonates with Ebstein’s malformation. Antegrade pulmonary blood
flow across a patent pulmonary valve may not occur in the presence of a PDA, resulting in functional pulmonary atresia. In a subset of patients with functional atresia and adequate RV systolic function, antegrade flow may resume following ductal closure. A patient with PDA and pulmonary regurgitation may present with extreme circulatory failure, and expeditious ligation of the ductus arteriosus abrogates the circular shunt. A sternotomy approach for ligation of the PDA allows access for cardiopulmonary bypass in case of circulatory collapse from inadequate right heart structures. A neonate with Ebstein’s malformation may present with circulatory failure or extreme cyanosis, warranting surgical intervention. In these patients, biventricular repair with TV repair alone carries a high mortality, and single-ventricle palliation with RV exclusion should be considered.
flow across a patent pulmonary valve may not occur in the presence of a PDA, resulting in functional pulmonary atresia. In a subset of patients with functional atresia and adequate RV systolic function, antegrade flow may resume following ductal closure. A patient with PDA and pulmonary regurgitation may present with extreme circulatory failure, and expeditious ligation of the ductus arteriosus abrogates the circular shunt. A sternotomy approach for ligation of the PDA allows access for cardiopulmonary bypass in case of circulatory collapse from inadequate right heart structures. A neonate with Ebstein’s malformation may present with circulatory failure or extreme cyanosis, warranting surgical intervention. In these patients, biventricular repair with TV repair alone carries a high mortality, and single-ventricle palliation with RV exclusion should be considered.
Adolescents and Adults
A patient who survives the neonatal period may remain relatively asymptomatic for years, even with significant tricuspid regurgitation. Indications for intervention include development of symptoms—specifically exercise intolerance, cyanosis, or arrhythmias. Timing of repair in the asymptomatic patient is controversial, but the presence of left ventricle compression, progressive right atrial (RA) or RV dilation by noninvasive imaging studies may herald onset of symptoms.
 SURGICAL TECHNIQUES
SURGICAL TECHNIQUESSeveral techniques have been described for TV repair. Original procedures developed for this abnormality involved posterior tricuspid annuloplasty and transverse or longitudinal plication of the atrialized portion of the RV, thereby bringing the true annulus to the level of the functional annulus. A significant portion of these patients subsequently require TV replacement. The operation described by da Silva et al. termed “cone reconstruction” has been most recently adapted by many pediatric centers. The procedure bears similarities to the technique described in 1988 by Carpentier, but differs in the extent of mobilization and rotation of leaflet apparatus. The components of the tricuspid repair include leaflet reconstruction, RV reduction by plication or resection, RA reduction, and creation of atrial lesions to prevent arrhythmias. Augmentation of deficient leaflets and papillary muscle repositioning may be necessary as well. Adjunct procedures include maintenance of a patent foramen ovale, creation of superior cavopulmonary shunt, and replacement of the pulmonary valve.
“Cone” Reconstruction of Tricuspid Valve
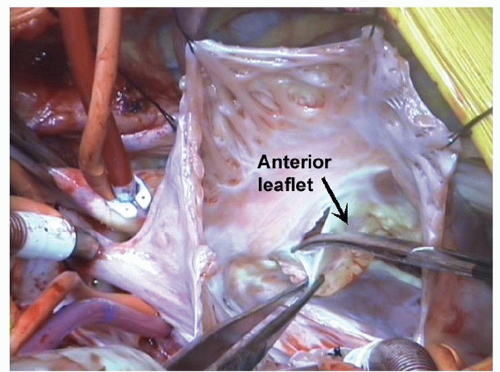
The goal of leaflet reconstruction is to utilize redundant valvular tissue from the anterior and posterior leaflets of the TV to re-create a new septal leaflet, which is appropriately located at the true annulus of the TV. The TV in Ebstein’s malformation may contain adequate surface area of leaflet material resulting from a large annular diameter and anterior leaflet circumference. Regurgitation results from maldistribution of leaflet material, such that the height of the posterior and septal leaflets is inadequate to coapt centrally with the anterior leaflet. The goal of the cone reconstruction is to redistribute existing leaflet tissue to the true annulus in the posterior and septal regions. Edge-to-edge reconstruction of leaflet material yields a circumferential leaflet of adequate height but smaller circumference, which allows for central coaptation. The leaflet reconstruction can be subdivided into discrete phases.
1. Detachment of leaflets and delamination of leaflet material. A circumferential incision is made in the anterior leaflet near its hinge point to the annulus, beginning at the anteroseptal commissure, extending clockwise onto the posterior leaflet and further onto the septal leaflet if present. Identification of the true annulus may be challenging due to tethering of leaflet tissue to the ventricle. In patients with type D malformation, there may be failure of delamination of the anterior leaflet tissue from underlying RV, giving an echocardiographic appearance of absent leaflet tissue. However, careful examination reveals distinct layer of leaflet tissue that can be delaminated from the RV mass. The distinction between leaflet and annulus is most obvious in the mid-portion of the anterior leaflet, and therefore, a circumferential incision is started at this point and carried in the clockwise and counterclockwise directions (Fig. 101.1). The hinge point to the annulus becomes easier to identify once delamination has been initiated on the anterior leaflet. Care must be exercised during delamination to avoid injury to the underlying myocardium or leaflet material itself. The leaflet detachment process is continued clockwise onto the posterior leaflet and septal leaflets, harvesting as much usable leaflet tissue as possible (Fig. 101.2). By delamination of fused leaflets, sufficient quantities of leaflet material can be frequently harvested to create a new TV without addition of prosthetic material.
2. Division of tethering secondary chords and muscle bundles. To allow adequate mobility of the leaflets for a cone-type reconstruction, the secondary chordal attachments and tethering muscle bundles must be mobilized down to the apex of the RV. These can be adequately visualized once the base of the leaflet has been detached from the tricuspid annulus (Fig. 101.3).




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree