Early Mobilization of Patients in the ICU
INTRODUCTION
Improvements in diagnosis and resuscitation of critically ill patients have prompted investigation into the burden of “survivorship.”1–5 Observational research has described substantial morbidity in survivors of critical illness, including general deconditioning, muscle weakness, dyspnea, depression, anxiety, and reduced health-related quality of life.6 A major catalyst for widespread attention was the comprehensive observations on a cohort of survivors of acute respiratory distress syndrome (ARDS).7 In this case series, patients were young and generally healthy prior to ARDS, and they experienced severe illness with prolonged critical care. Despite severe acute lung injury, serial follow-up examinations demonstrated that lung function generally normalized during the first year after ICU discharge. In contrast, all patients reported poor function attributed to the loss of muscle bulk, proximal limb weakness, and fatigue. Patients exhibited impaired endurance, and only 49% of patients had returned to work. At 5 years after ICU discharge, subjective weakness and decreased exercise capacity continued.8 Although 77% of patients were working by the fifth year, patients often required a modified work schedule, gradual transition back to work, or job retraining. In addition, patients were plagued with psychological illness. More than half of survivors experienced at least one episode of depression or anxiety.
Other investigations have reported similar findings of post-ARDS debilitation.9,10 An observational trial measured a 66% cumulative incidence of physical impairment during 2-year follow-up.9 The impairment, defined as the acquisition of two or more dependencies in instrumental activities of daily living, had greatest incidence by 3 months after discharge and was associated with longer ICU stay and prior depressive symptoms.
Acquired neuromuscular weaknesses, loss of function, and cognitive impairment have been measured in other critical care settings, such as in severe sepsis and during mechanical ventilation in the elderly. For example, the morbidity of a hospitalization for severe sepsis was evaluated utilizing a registry of Americans over age 50 years who underwent biennial evaluations of cognitive and physical function.11 Among patients with no functional limitations at baseline, hospitalization for severe sepsis was associated with the development of 1.57 new limitations, as well as a more rapid rate of development of functional limitations after hospitalization. In addition, the incidence of severe sepsis was highly associated with progression to moderate-to-severe cognitive impairment. Similar acquired disability has been observed in a longitudinal study of elderly patients undergoing hospitalizations that included need for mechanical ventilation.12 In adjusted analyses, mechanical ventilation was associated with a 30% greater disability in activities of daily living and a 14% greater disability in mobility.
These studies show that decrements in physical function occur across the spectrum of critical illness. Although outcomes may be influenced by other factors, such as age, pre-existing comorbidities, acquired psychological and cognitive dysfunction, and social support, it is clear that weakness needs to be recognized early to enable preventive interventions.
ICU-ACQUIRED WEAKNESS
Many patients admitted to the ICU develop a syndrome of neuromuscular dysfunction characterized by generalized muscle weakness and an inability to be liberated from mechanical ventilation. Since this syndrome occurs in the absence of preexisting neuromuscular disease, it is believed to reflect illnesses or treatments occurring in the ICU. Early reports described two categories of acute, acquired neuromuscular dysfunction: polyneuropathy and myopathy.13–15 Decades of research on this acquired nerve and muscle injury has characterized specific phenotypes with respect to comprehensive physical examination findings, electrophysiologic testing results, and muscle and nerve histopathology. Overall, the spectrum of neuromuscular disorders acquired in the ICU is now collectively referred to as “ICU-acquired weakness” (ICUAW).16
Several studies have attempted to establish the prevalence of ICUAW and its associated risk factors. To date, the best summary data are outlined in a systematic review of 24 published studies that included both clinical and electrophysiologic characterizations of the weakness.17 Of 1421 total patients with sepsis, multiorgan failure, or prolonged mechanical ventilation, 46% had ICUAW. The risk of ICUAW was associated with hyperglycemia (and inversely associated with tight glycemic control), the systemic inflammatory response syndrome (SIRS), sepsis, multiple organ dysfunction, renal replacement therapy, and catecholamine administration. Across studies, there was no consistent relationship between ICUAW and patient age, gender, severity of illness, or medication exposure. Similar findings have been found in a seminal observational study describing ICUAW.18 In this study of patients undergoing seven or more days of mechanical ventilation, 25% experienced ICUAW. Independent predictors of ICUAW included the number of days with multiple organ dysfunction and the duration of mechanical ventilation. In contrast to the systematic review, female sex and administration of corticosteroids were strong predictors of ICUAW.
Unfortunately, few modifiable risk factors for ICUAW have generated opportunities to improve outcomes. Prospective, randomized trials focusing on exposure to corticosteroids and neuromuscular blocking agents have demonstrated neither consistent nor conclusive results for an association with weakness.19–21 A Cochrane review identified only one successful preventive intervention for ICUAW: insulin therapy with strict glycemic control.22 This finding, based on two, single-center, randomized trials, has been challenged by results from subsequent multicenter trials. Specifically, recent trials of intensive insulin therapy have been associated with an increased risk of severe hypoglycemia and either increased or unchanged mortality rates when compared to more permissive blood glucose ranges.23,24 Accordingly, this treatment option has not been embraced.
For all forms of ICUAW, care is supportive and includes aggressive management of sepsis and underlying medical conditions. Because prolonged immobilization and bed rest have been shown to accelerate muscle loss, early mobilization has emerged as a potential preventive measure. Although no one has systematically measured immobility during ICU care, clinicians acknowledge its occurrence during the earliest days of critical illness, particularly during deep sedation or neuromuscular blockade, specific mechanical ventilation strategies (e.g., prone ventilation), and other advanced support (e.g., continuous hemodialysis).
THE CONSEQUENCES OF BED REST AND INACTIVITY
Rest is necessary for the repair of weak or damaged tissue and muscle remodeling. Prolonging rest has potential benefit, such as avoiding pain in the injured body part and permitting maximal utilization of metabolic resources for healing.25 Reducing oxygen consumption, minute ventilation needs, and cardiac demand via rest during critical illness may be logical. However, investigations have found few studies that demonstrate even modest benefits from extending bed rest. In contrast, trials of rheumatoid arthritis, low back pain, uncomplicated myocardial infarction, pulmonary tuberculosis, and deep venous thrombosis have demonstrated improved outcomes when bed rest was limited or avoided.26
Prolonged physical inactivity has been modeled through investigations of space flight, immobilization of a limb, lower limb suspension, and bed rest.25,27 Each of these modalities demonstrate that muscle mass, as assessed using computed tomography or magnetic resonance imaging, decreases by approximately 1.5% to 2.0% per day during the first 2 to 3 weeks of enforced rest. Measures of muscle strength demonstrate weakness that parallels the changes in muscle size. For example, individual studies have shown knee extensor strength declined by 22% after 14 days and by 53% after 28 days of limb immobilization.28,29 Limb casting models of immobilization demonstrate declines in strength as high as 5% to 6% per day.30,31 The antigravity muscle groups – located in the legs, trunk, and neck – experience the most pronounced muscle atrophy.
Limb injuries of prolonged inactivity also include loss of joint range of motion and muscle contractures. One study of survivors of a 2-week or longer critical illness found that joint contractures occurred in 39% of patients.32 At the time of discharge from intensive care, 34% of patients had at least one functionally significant contracture, and 23% of patients had functionally significant contractures persisting at the time of discharge. The most commonly affected joints at the time of discharge were the elbow (34%) and ankle (33%).
Other injuries associated with prolonged bed rest and inactivity include pressure ulcers, atelectasis, and thromboembolic disease.25 Indolent effects, such as endocrinopathy and cardiovascular function, have also been described. For example, healthy volunteers undergoing bed rest demonstrate insulin resistance and increases in total cholesterol and triglycerides.33 Orthostatic intolerance from baroreceptor dysfunction is commonplace; studies demonstrate increases in systemic vascular resistance following bed rest. For the patient in the ICU, superimposing these developments on existing critical illness may be costly with respect to both survival and survivorship.
MOBILIZATION OF THE CRITICALLY ILL PATIENT
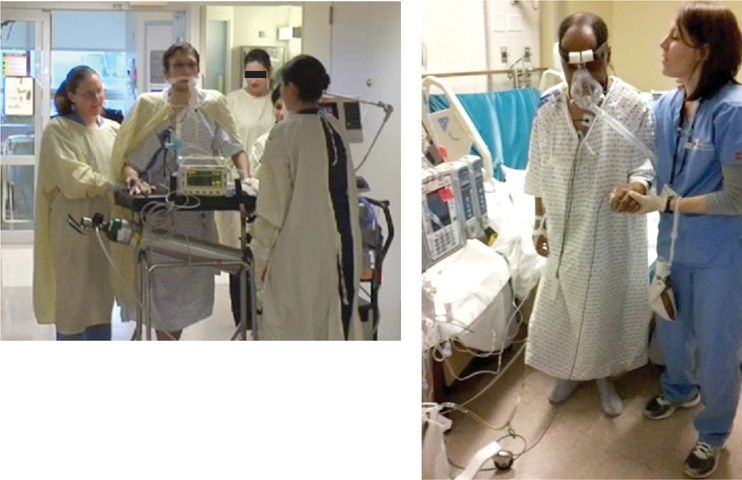
Mobility has long been recognized as a measure to hasten recovery. Given the commonality of ICUAW and the potential for immobility in routine critical care, particularly during mechanical ventilation, investigators have targeted early mobilization as a potential opportunity to improve strength and functional outcomes in survivors (Fig. 151-1). In summary, recent trials highlight that early exercise and mobilization are safe to conduct despite ongoing critical illness, improve patient functional outcomes, and shorten ICU and hospital lengths of stay.34 Although most investigations have focused selectively on patients undergoing mechanical ventilation, the results are likely generalizable to broader populations of critically ill patients.
Figure 151-1 A. A patient with cystic fibrosis, pneumonia, and respiratory failure ambulating in ICU on day 5 while receiving invasive mechanical ventilation via an oral endotracheal tube. He is accompanied by his nurse, respiratory therapist, physical therapist, and wife. B. Patient with exacerbation of chronic obstructive pulmonary disease and pneumonia marching in place while undergoing noninvasive ventilation under the direction of a physical therapist.
 PASSIVE RANGE OF MOTION
PASSIVE RANGE OF MOTION
Passive range of motion exercise is conducted to preserve the range of the joint. Passive range of motion exercise is an expectation of routine care, yet often overlooked. However, evidence to support the isolated use of passive movement is weak. Limited evidence suggests that it may prevent protein degradation, maintain muscle mass, and alter the inflammatory profile in humans. For example, in 20 subjects with severe sepsis or septic shock randomized to 30 minutes of predominantly passive exercise or no intervention, the group receiving passive exercise demonstrated preserved fat-free mass, decreased IL-6 and increased IL-10 levels compared with control patients, who lost 7% of fat-free mass in the first 7 days following admission to the ICU.35
One study examined whether muscle wasting in critically ill patients could be prevented with stretching alone.36 Continuous passive motion was administered by a machine over 7 days to one leg of five separate critically ill adults. In the muscles that received continuous passive stretch, smaller reductions in muscle fiber cross-sectional area and protein per gram of wet muscle weight were noted. However, clinical observation suggests that more than simple passive movement is necessary to help preserve muscle strength.
 ACTIVE RANGE OF MOTION
ACTIVE RANGE OF MOTION
“Early mobilization” is defined as the intensification and early application of physical and occupational therapy administered to critically ill patients. The safety and feasibility for early mobilization during mechanical ventilation was first captured in a descriptive cohort study published in 2007.37 This single-center study in a respiratory ICU tracked the activity levels of 103 patients over an average of 10 days following inception of critical illness. The exercise program began once patients responded to verbal stimulation and exhibited stable respiratory and cardiovascular function (defined as FIO2 ≤0.6, PEEP ≤10 cm H2O, and absence of orthostatic hypotension and catecholamine drips). The exercise team, including physical therapist, respiratory therapist, nurse, and critical care technician, focused training on three activities: sitting on the edge of the bed, sitting in a chair after bed transfer, and ambulation. Maximal activity level was tracked. For this cohort, ICU measurements at discharge showed that 77% of patients were able to ambulate (including 69% able to ambulate >100 ft), 15% of patients were able to sit in a chair, and 5% of patients were only able to sit at the edge of the bed. Most importantly, only 14 of the 1449 activity events resulted in predefined adverse events. Specifically, there were five falls to the knees without injury, four drops in systolic blood pressures to <90 mm Hg, one systolic blood pressure rise to >200 mm Hg, three oxygen desaturations to <80% saturation, and one nasal feeding tube dislodgement.
To emphasize the differences in their practice of early mobilization compared to other ICUs, the same investigators studied the performance levels of mechanically ventilated patients within a 2-day window before and after transfer to the ICU.38 Within 24 hours of arrival, patients successfully underwent more intense physical activities. For example, ambulation increased from 11% pretransfer to 41% within 48 hours. Multivariable logistic regression demonstrated that transfer to the therapy-dominant ICU was independently associated with the likelihood of ambulation. This study was the first to demonstrate that a unit-based culture of early mobilization could significantly influence patient functional performance.
The first prospective trial comparing early exercise and mobilization with usual care was published in 2008.39 In this study, a mobility team, including a physical therapist, nurse, and nurse assistant, implemented stepwise increases in therapy based on patient participation and tolerance. Therapy spanned passive range of motion (ROM) to active ROM exercise, sitting, transfers, and ambulation. Therapy practices between groups were strikingly different: 80% of intervention patients underwent at least one therapy session compared with only 47% of patients in the usual care group. Intervention patients were bedridden for a shorter period (8.5 vs. 13.7 days) and had a reduced hospital length of stay (14.9 vs. 17.2 days). Recently, longer-term outcomes of the initial 330 patient cohort were reported.40 In multivariate analysis, the lack of early ICU mobility was independently associated with readmission(s) or death during the first year. Although the etiology for readmission and death was not specified, these findings suggest early ICU mobility may have a sustained effect.
In 2009, a prospective, dual-center, randomized, clinical trial of very early mobilization was published.41 One hundred and four patients in medical ICUs were enrolled within 72 hours of the onset of respiratory failure requiring mechanical ventilation. Patients were randomized to an intervention group that received mandated, progressive physical and occupational therapy, or a control group with therapy services ordered by the primary team. The dual therapist team treated patients daily with progressive mobilization, including bed exercises, sitting at the edge of the bed, simulation of activities of daily living, transfer training, and ambulation. In the intervention group, patients underwent therapy on 87% of days, beginning 1.5 days after intubation, compared to 7 days in the control group. Within 4 days, 76% of intervention group were sitting at the edge of the bed, 33% were standing and transferring to a chair, and 15% were ambulating. At hospital discharge, patients in the intervention group had a higher rate of return to independent functional status (59% vs. 35%), greater independent walk distance, and were more likely to be discharged to home (43% vs. 24%). In addition, patients in the intervention group experienced a reduced duration of delirium (2 vs. 4 days) and more ventilator-free days (23.5 vs. 21.1 days), but no significant difference in ICU or hospital length of stay.
Finally, combining the interventions of sedation minimization and early mobilization may yield the most pronounced benefit for patients with respiratory failure. This was demonstrated by a quality improvement project conducted in 2010 in a tertiary academic center.42 In the preintervention phase, mechanically ventilated patients were deeply sedated during 58% of all patient-days and were either deeply sedated or delirious on more than 85% of all patient-days. Only 24% of patients had consultations for PT or OT while in the medical ICU. Early mobilization stakeholders implemented the following changes: education on sedation and mobilization practices, augmentation of therapist staffing, promotion of physiatry and neurology consultation, and provision of regular feedback to clinicians on these practices. In the postintervention period, patients were more likely to be awake without delirium, receive more therapy services, and exhibit improved functional mobility. In addition, administrative data on all patients in the medical ICU demonstrated reductions in lengths of stay in the ICU (2.1 days) and hospital (3.1 days).
 USE OF ASSISTIVE TECHNOLOGIES
USE OF ASSISTIVE TECHNOLOGIES
Assistive technologies offer the opportunity to begin physical activity at the earliest phases in critical illness. So far, two techniques have been studied most extensively: cycle ergometry and neuromuscular electrical stimulation. Both interventions offer the promise of muscle engagement in the noninteractive patient.
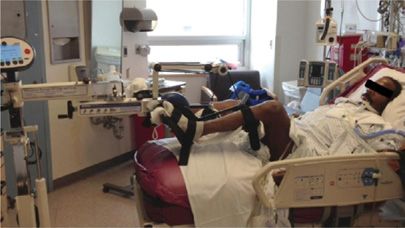
The cycle ergometer is a stationary cycle that can be positioned above the foot of the bed (Fig. 151-2). The device can alter the amount of work performed by the patient, including passive cycling for the comatose patient. Engaged patients can actively pedal with varying resistance. In healthy subjects undergoing prolonged immobilization, this cycling has been shown to preserve thigh muscle thickness.43 In addition, the technique has been proved to be both feasible and safe during hemodialysis and for patients with chronic obstructive pulmonary disease.44,45
Figure 151-2 A 58-year-old man with chronic hemiparesis undergoing passive cycling on a cycle ergometer while undergoing invasive mechanical ventilation for acute respiratory distress syndrome complicated by severe sepsis and hypoactive delirium.
Multimodality mobilization, combining cycle ergometry and standard physical therapy, has been tested in a single-center randomized trial.46 Ninety patients with ICU stays greater than 5 days were randomized to either multimodality therapy or standard physical therapy alone. Patients receiving the intervention underwent cycling sessions conducted 5 days per week, lasting approximately 30 minutes per session. The study demonstrated safety, feasibility, and efficacy. Of the 425 cycling sessions, no serious adverse events were noted. Only 4% of sessions resulted in early termination due to oxygen desaturation or significant changes in blood pressure. At hospital discharge, patients in the intervention group exhibited a longer 6-minute walk distance, higher survey scores on physical function, and greater increases in quadriceps force.
The second technique, neuromuscular electrical stimulation (NMES), creates nonvolitional contraction of skeletal muscles. Skin surface electrodes deliver low-voltage electrical impulses to underlying muscle. NMES has been studied in outpatients with chronic heart failure and chronic obstructive lung disease to preserve or improve muscle mass, exercise capacity, and function.47 Despite the promise of the technique, randomized trials in critical illness have reported conflicting results. Six unique ICU trials in patients with acute respiratory failure and sepsis and a trial in patients receiving chronic mechanical ventilation demonstrated mixed, but promising results for potential efficacy.48 For example, the largest study of critically ill patients randomized 140 patients to NMES versus standard care. Sessions of transcutaneous lower extremity muscle stimulation were conducted daily for 1 hour.49 Patients in the intervention arm exhibited higher static muscle strength sum scores compared with controls. However, concerns over endpoint selection, measurement bias, and the need to assess patient tolerance have generated a call for further investigation prior to widespread implementation.
PRACTICAL IMPLEMENTATION OF AN EXERCISE AND MOBILIZATION PROGRAM IN AN ICU
To translate early exercise and mobilization to the acute care ICU, programs must adapt to balancing unpredictable acute care management with the desire to keep rehabilitation a priority. Competing challenges include patient factors (such as delirium and pain), critical care procedures (such as dialysis and bedside procedures like vascular catheter insertion), and clinician factors (such as biases about safety and rehabilitation).
Maximal patient engagement requires optimal management of pain, agitation, and delirium. Protocols to guide drug sedative and analgesic administration may be a necessary prerequisite. In accordance with guidelines, hallmarks of such programs include the utilization of a reproducible, validated scale (e.g., Richmond Agitation and Sedation Scale), an established sedation target prescribed daily, and nurse-led titration of drug administration.50 Although recent research has questioned the necessity of daily interruption of continuous sedative and analgesic infusions, all experts agree that methods to aggressively minimize drug administration to a “least necessary” model are optimal.51
For the mechanically ventilated patients, some experts have advocated the bundling of early mobilization with programs that include spontaneous awakening trials, spontaneous breathing trials, and delirium screening. This bundle, termed “ABCDE,” stands for Awake and Breathing Coordination, Delirium screening, and Early mobility.52 The individual components are based upon clinical trials of benefit (excluding delirium screening) with improved outcomes, such as reductions in ICU and hospital lengths of stay and duration of mechanical ventilation, as well as an association with improved mortality at 1 year.41,53,54 Clinical researchers are now assessing the feasibility of broad implementation of the initiative.55
Criteria to guide initiation of therapy are similarly important. Observational and interventional trials to date have used criteria focusing on the cardiovascular, pulmonary, and neurologic systems to identify candidate ICU patients (Table 151-1).37,39,42 However, these criteria have been developed for patients undergoing mechanical ventilation and may need further additions as broader patient populations are engaged (e.g., patients with recent gastrointestinal bleeding or surgery). In contrast, veteran critical care therapists may find these criteria too conservative. Data has shown that mobilization may occur in contexts of greater ventilator or oxygen dependence, and patients with advanced delirium or coma may engage in exercise through the use of assistive devices.56