Double Outlet Ventricles
Kirk R. Kanter
DOUBLE-OUTLET RIGHT VENTRICLE
Definition
Double-outlet right ventricle (DORV) refers to a heterogeneous group of cardiac malformations characterized by an abnormal ventriculoarterial connection in which both great arteries are related to the right ventricle. Although the term double-outlet right ventricle can be correctly applied to hearts with atrioventricular discordance (e.g., congenitally corrected transposition of the great arteries) or to hearts with univentricular atrioventricular connections (e.g., double-inlet left ventricle), for simplicity of discussion, only hearts with atrioventricular concordance and two adequate ventricles are discussed in this chapter.
The actual definition of what constitutes a DORV has been the source of controversy in the literature. Although some have required the presence of bilateral infundibula or atrioventricular valve-semilunar valve discontinuity (most commonly mitral-aortic discontinuity), these criteria are not essential in establishing the diagnosis of DORV. From a surgical perspective, it is most useful to adopt the “50% rule” in defining DORV. With this rule, a heart is termed DORV if >50% of both great arteries arise from the right ventricle. Usually, all of one great artery and ≥50% of the other great artery arise from the right ventricle in DORV.
Classification
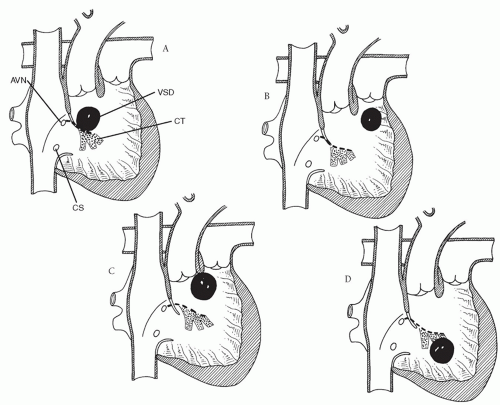
A ventricular septal defect (VSD) is almost always present with DORV. Based on the work of Lev and colleagues, DORV is classified into four groups based on the relationship of the VSD to the great arteries (Table 88.1): subaortic, subpulmonary, doubly committed, and noncommitted (remote).
Subaortic Ventricular Septal Defect
DORV with subaortic VSD (Fig. 88.1A) is the most common group of DORV. It may or may not be associated with pulmonary stenosis. The presentation without pulmonary stenosis is similar to that of a child with a large VSD (heart failure). If there is pulmonary stenosis, it is usually infundibular, so the clinical presentation in this subgroup is similar to that of tetralogy of Fallot (cyanosis, hypercyanotic episodes). Accordingly, if a patient is not a candidate for complete repair at the time of presentation because of size, clinical condition, or other variables, palliative procedures in DORV with subaortic VSD without pulmonary stenosis would be pulmonary artery banding; in children with pulmonary stenosis, a systemic-to-pulmonary artery shunt would be appropriate.
Subpulmonary Ventricular Septal Defect
DORV with subpulmonary VSD (the Taussig-Bing anomaly) is the second most common group of DORV (Fig. 88.1B). Because of the location of the VSD, oxygenated left ventricular blood preferentially streams through the VSD into the pulmonary artery and desaturated right ventricular blood streams into the aorta as in transposition of the great arteries with VSD. These children present with cyanosis and heart failure. Associated coarctation of the aorta occurs commonly; aortic arch obstruction and subaortic stenosis can also be associated with the Taussig-Bing anomaly. Because DORV with subpulmonary VSD is prone to early development of pulmonary vascular obstructive disease, intervention in infancy is usually necessary. In addition to repair of the aortic coarctation and arch hypoplasia, if present, balloon atrial septostomy is commonly needed to improve mixing of oxygenated blood at the atrial level as in transposition of the great arteries. Although palliation can be accomplished with pulmonary artery banding, it is preferable to proceed with complete repair in infancy.
Doubly Committed Ventricular Septal Defect
In DORV with doubly committed VSD, the VSD is immediately beneath both the pulmonary artery and the aorta (Fig. 88.1C). Usually, the infundibular septum is absent or hypoplastic. As in DORV with subaortic VSD, there may be associated pulmonary stenosis. Thus, clinical presentation and surgical approach would be similar to that for DORV with subaortic VSD with or without pulmonary stenosis.
Noncommitted Ventricular Septal Defect
In the final group of DORV, the VSD is committed neither to the aorta nor to the pulmonary artery (Fig. 88.1D). The remote location of the VSD may be in the inlet septum as with atrioventricular septal defects or a muscular VSD in the trabecular septum. Pulmonary stenosis may be present. The presentation and surgical palliation of DORV with noncommitted VSD would be similar to that for DORV with subaortic VSD with or without pulmonary stenosis.
Surgical Techniques
The goal of surgical repair in DORV is to achieve a biventricular repair utilizing the left ventricle as the systemic ventricle with unobstructed right and left ventricular outflow tracts. Usually, this can be accomplished within the first 6 to 12 months of life, thus obviating the need for palliative procedures. If it is anticipated that the final repair will require an extracardiac valved conduit or a complicated intraventricular tunnel, it is reasonable to delay correction to allow for growth by palliating, if necessary, with a pulmonary artery band or a systemic-to-pulmonary artery shunt. Also, in children who eventually will need a Fontan procedure, it is critical to protect the pulmonary vascular bed early on with appropriate palliation.
Table 88.1 Classification of Double-Outlet Right Ventricle | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Roughly 10% of children with DORV will have a restrictive VSD. By definition, this means that outflow from the left ventricle is obstructed, and therefore, early repair with enlargement of the VSD is mandated since spontaneous closure of the VSD, rather than being curative as in isolated VSD, would be fatal in DORV. Pulmonary artery banding is certainly not appropriate in patients with DORV and a restrictive VSD.
Intraventricular Tunnel Repair of Double-Outlet Right Ventricle with Subaortic Ventricular Septal Defect without Pulmonary Stenosis
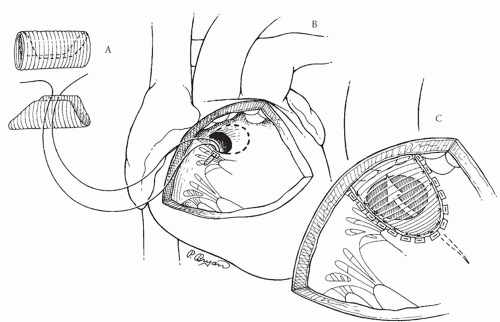
Repair of DORV with a subaortic VSD is accomplished by creating an intraventricular tunnel channeling left ventricular blood through the VSD to the aorta (Fig. 88.2). This is facilitated by the use of a polyester (Dacron) or collagen-impregnated polyester tube graft corresponding to the size of the aorta. This is opened longitudinally so that about two-thirds of its circumference is available (Fig. 88.2A). The advantage of using a tube graft for the intraventricular tunnel is that the corrugations keep the required curve in the baffle to allow unobstructed left ventricular outflow. A flat Dacron or polytetrafluoroethylene (Gore-Tex) patch that one would use to close a simple VSD would be prone to kinking
and would create obstruction unless the geometry and the size of the patch were exactly perfect.
and would create obstruction unless the geometry and the size of the patch were exactly perfect.
After routine establishment of cardiopulmonary bypass with bicaval cannulation and cardioplegic arrest, the intracardiac anatomy is carefully inspected through a right atriotomy. The VSD is visualized through the tricuspid valve and its relationship to the aorta is confirmed. If there is any suspicion preoperatively or intraoperatively that the VSD is smaller than the aorta, it should be enlarged. This can be accomplished through the tricuspid valve or the right ventricle with either a transverse or a longitudinal right ventriculotomy. The VSD is enlarged superiorly and anteriorly (Fig. 88.2B), thus resecting some of the infundibular septum. Enlarging the VSD posteriorly and superiorly through the ventriculoinfundibular fold runs the risk of going outside the heart. The conduction tissue runs inferiorly and of course should be avoided (Fig. 88.1A).
Once the VSD is enlarged (if necessary), the Dacron tube graft is oriented so that the longitudinal axis of the graft corresponds to an imaginary line from the anterior-most portion of the aorta to the anterior-inferior limit of the VSD (Fig. 88.2C). It is helpful to place the first suture through the base of the tricuspid valve leaflet at the anteroseptal commissure, and then through the midportion of the tube graft (Fig. 88.2B). About one-third of the VSD sutures are placed along the posterior and inferior rim of the defect through the tricuspid valve, with care taken to avoid the conduction tissue, with several of these pledgetted sutures on the atrial side of the septal leaflet of the tricuspid valve. These sutures are passed through the Dacron tube graft, which is seated down and the sutures tied. The remainder of the circumference of the VSD is closed through the right ventriculotomy, with care being taken to maintain proper orientation of the patch (Fig. 88.2C). Anteriorly and superiorly, it may be advantageous to anchor some of the pledgetted sutures through the anterior wall of the right ventricle above the aortic valve, as is done in a Rastelli procedure. Rather than using interrupted pledgetted sutures, the VSD can be closed with a continuous suture technique completely through the right ventriculotomy.
If it appears that the intraventricular tunnel is bulging into the right ventricular outflow tract, the right ventriculotomy is closed with a patch of autologous pericardium to avoid right ventricular outflow tract obstruction.
Repair of Double-Outlet Right Ventricle with Subaortic Ventricular Septal Defect and Pulmonary Stenosis
In the group of patients with DORV with subaortic VSD and pulmonary stenosis, it is useful to mark the planned right ventriculotomy incision with stay sutures before cardioplegic arrest after carefully identifying the course of the epicardial coronary arteries (Fig. 88.3A). The intraventricular tunnel repair of the VSD is identical to that used for patients with DORV and subaortic VSD without pulmonary stenosis (Fig. 88.3B). If a major coronary artery crosses the right ventricular outflow tract or if the pulmonary vascular resistance is elevated or if there is distal pulmonary arterial obstruction, a valved conduit should be used to establish right ventricle-pulmonary artery continuity. The pulmonary trunk is divided, with the proximal main pulmonary artery oversewn (Fig. 88.3B). The proximal portion
of a valved homograft is sewn to the superior aspect of the right ventriculotomy. The homograft is trimmed to the proper length, and an end-to-end anastomosis between the distal homograft and the distal main pulmonary artery (or pulmonary bifurcation if the main pulmonary artery is small) is performed. Finally, the gap between the proximal right ventriculotomy and the proximal homograft is roofed with left-over homograft material or autologous pericardium (Fig. 88.3C). Alternatively, the native pulmonary trunk can be left intact, and the distal homograft conduit is sewn end-to-side to the junction of the native pulmonary trunk and pulmonary bifurcation allowing blood flow through both the stenotic native pulmonary valve and the new conduit.
of a valved homograft is sewn to the superior aspect of the right ventriculotomy. The homograft is trimmed to the proper length, and an end-to-end anastomosis between the distal homograft and the distal main pulmonary artery (or pulmonary bifurcation if the main pulmonary artery is small) is performed. Finally, the gap between the proximal right ventriculotomy and the proximal homograft is roofed with left-over homograft material or autologous pericardium (Fig. 88.3C). Alternatively, the native pulmonary trunk can be left intact, and the distal homograft conduit is sewn end-to-side to the junction of the native pulmonary trunk and pulmonary bifurcation allowing blood flow through both the stenotic native pulmonary valve and the new conduit.
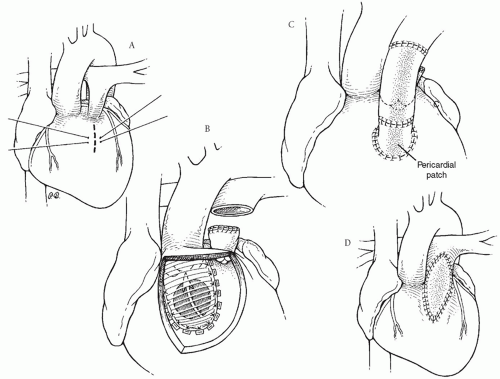 Fig. 88.3. Repair of double-outlet right ventricle with subaortic ventricular septal defect and pulmonary stenosis. (A) The site of the proposed right ventriculotomy is shown by a dashed line. Marking sutures are placed before cardioplegic arrest to aid with retraction and to properly orient the ventricular incision. (B) An intraventricular tunnel is fashioned as in Figure 88.2. The main pulmonary artery is divided and the cardiac end oversewn. (C) Right ventricle-to-pulmonary artery continuity is reestablished with a valved homograft conduit. About one-half of the circumference of the proximal homograft is sutured to the superior aspect of the right ventriculotomy. A piece of pericardium or homograft material is used to roof the gap between the right ventricle and the homograft. (D) Alternatively, a nonvalved transannular right ventricular outflow tract patch can be used to relieve the pulmonary stenosis. |
Often it is possible to establish an adequate right ventricular outflow tract without a valved conduit as is done with repair of tetralogy of Fallot. In this case, after the division of obstructing right ventricular muscle bundles, a transannular outflow tract patch is created using autologous pericardium (Fig. 88.3D). If the pulmonary valve annulus is of good size, a nontransannular patch is adequate after a pulmonary valvotomy is performed. Otherwise, a transannular patch is necessary.
Sakamoto and colleagues have described an interesting transaortic approach for enlarging the VSD and tunneling left ventricular outflow through the VSD to the aorta in two patients with DORV with subaortic VSD and pulmonary stenosis.
Anatomic Repair of Double-Outlet Right Ventricle with Subpulmonary Ventricular Septal Defect
Currently, the preferred surgical repair of DORV with subpulmonary VSD (the Taussig-Bing anomaly) is the anatomic repair (the arterial switch operation). Since coarctation of the aorta is commonly seen in this group, these patients may have had prior coarctation repair with a pulmonary artery band, although simultaneous repair of the coarctation at the time of anatomic repair is now the accepted standard.
The VSD is closed either through a right atriotomy or through a right ventriculotomy channeling left ventricular blood into the pulmonary artery (Fig. 88.4A). The aorta is transected slightly higher than the pulmonary trunk (Fig. 88.4A). If present, the coarctation and associated aortic arch hypoplasia can be corrected at this time with a patch of homograft material using a brief period of profound hypothermia with circulatory arrest or regional low flow perfusion.
Particularly with side-by-side great arterial relationships, the circumflex coronary artery often arises with the right
coronary artery from the right posterior-facing sinus, whereas the anterior descending coronary artery originates from the left posterior-facing sinus. In this situation, the anterior descending coronary artery is excised as a button and transferred to a defect created in the left ant-eriorfacing sinus of the proximal pulmonary trunk (neoaorta; Fig. 88.4B



coronary artery from the right posterior-facing sinus, whereas the anterior descending coronary artery originates from the left posterior-facing sinus. In this situation, the anterior descending coronary artery is excised as a button and transferred to a defect created in the left ant-eriorfacing sinus of the proximal pulmonary trunk (neoaorta; Fig. 88.4B
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree