TERMINOLOGY
The ventriculoarterial connection is considered “double-outlet” when more than 50% of each great artery arises from one ventricle. The origin of both great arteries from the right ventricle (RV) is termed double-outlet right ventricle (DORV). Conversely, the origin of both great arteries from the left ventricle (LV) is termed double-outlet left ventricle (DOLV).
DOUBLE-OUTLET RIGHT VENTRICLE
Basically recognized by the origin of both great arteries from the morphologic RV, DORV encompasses hemodynamic features of a variety of entities, ranging from simple ventricular septal defect (VSD) with excessive pulmonary blood flow, to tetralogy of Fallot (TOF) with reduced pulmonary artery flow secondary to pulmonary stenosis, to transposition of the great arteries (TGA) with systemic desaturation and pulmonary overcirculation. This congenital malformation is a rare anomaly. Its frequency has been reported as approximately 0.09 per 1000 births and represents 1% to 1.5% of patients with congenital heart disease. No racial/ethnic or sexual predilection is evident.
There are 16 possible variations of DORV based on the great artery relationships and the location of the VSD. Figure 17.1 illustrates these variations but shows that only nine types were observed clinically. However, this series includes only cases with situs solitus of the atria and viscera, atrioventricular (AV) concordance, and two well-developed ventricles and AV valves. The location of the VSD is described as subaortic, subpulmonary, doubly-committed (below both great arteries), noncommitted, or remote (Fig. 17.2). In addition, an intact ventricular septum (very rare) allows four other possible types of DORV, depending on the great artery relationships. Multiple other variations and combinations are possible if one also includes situs inversus and situs ambiguous, AV discordance, and AV valve atresia.
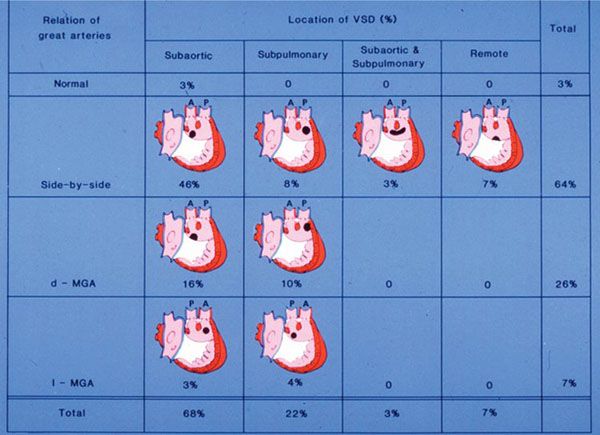
Figure 17.1. Relationships of the great arteries and locations of the ventricular septal defect (VSD) in 70 patients with double-outlet right ventricle (DORV). Ao, aorta; d-MGA, dextro-malposed great arteries; l-MGA, levo-malposed great arteries; P, pulmonary artery. (Reprinted with permission from Hagler DJ, Tajik AJ, Seward JB, et al. Double-outlet right ventricle: wide-angle two-dimensional echocardiographic observations. Circulation. 1981;63:419–428.)
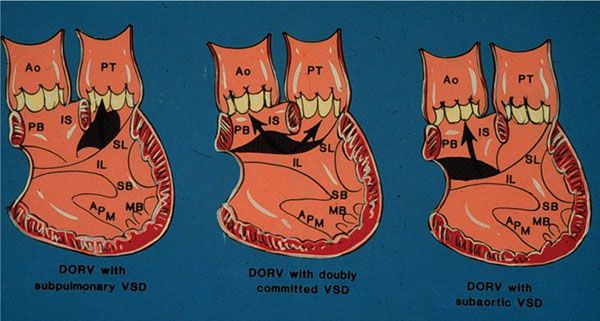
Figure 17.2. Schematic illustration of three ventricular septal defect (VSD) locations in double-outlet right ventricle. Ao, aorta; APM, apical papillary muscle; IL, inferior limb; IS, conus septum; MB, moderator band; PB, parietal band; PT, pulmonary artery; SB, septal band; SL, superior limb. (Courtesy of Dr. William Edwards, Mayo Clinic.)
Double-Outlet Right Ventricle with Side-by-Side Great Arteries and Subaortic Ventricular Septal Defect
This represents the most common and typical form of DORV. The presence of bilateral conus separates both semilunar valves from both AV valves. The VSD is the only outlet from the LV, and the aortic conus is a muscular structure between the aortic valve and the anterior leaflet of the mitral valve. Figure 17.3 illustrates the pathologic findings of this type of DORV. The RV has been opened illustrating both great arteries originating from the RV. Figure 17.4 illustrates the angiographic findings in a patient with DORV, subaortic VSD, and side-by-side great arteries.
Two-dimensional echocardiographic findings for the diagnosis of DORV have been reported. Three observations were noted for the diagnosis: (a) origin of both great arteries from the anterior RV, (b) mitral–semilunar valve discontinuity, and (c) absence of left ventricular outflow other than the VSD. In a series of 36 patients studied by two-dimensional echocardiography, both great arteries were observed to originate predominantly from the anterior RV. If one great artery overrode the VSD, nearly exclusive or predominant commitment to the RV was required for diagnosis. Figures 17.5, 17.6, and 17.7 illustrate the most common forms of DORV with side-by-side or normally related, right anterior aorta, and left anterior aorta, respectively. In addition, Figure 17.7 illustrates the associated finding of left-juxtaposed atrial appendages. Figure 17.8 illustrates the pathologic features of a patient with subpulmonary VSD with right anterior aorta. Most patients with DORV will have situs solitus of the atria and viscera, but situs ambiguous with bilateral right- or left-sidedness and situs inversus may be present. Most patients with DORV will have AV concordance, but less commonly AV discordance (ventricular inversion) may be present.
IMAGING PEARLS
Position of the Great Arteries
In two-thirds of the cases, both great arteries can be observed simultaneously originating from the anterior RV. In the subcostal sagittal plane, both semilunar valves may not be visualized simultaneously. However, each semilunar valve may be demonstrated with slight right or left transducer angulation. The semilunar valves are positioned in a more anterior and superior location relative to the rest of the ventricle because of conus muscle beneath the valves. It is important to sweep in the short-axis scans from apex to base to demonstrate the commitment of each great artery to the right ventricular cavity (Fig. 17.9). With the use of a short-axis scan at the cardiac base, a double-circle appearance of the great arteries is consistent with a parallel orientation of the great arteries. A superior short-axis scan demonstrates the pulmonary artery bifurcation.
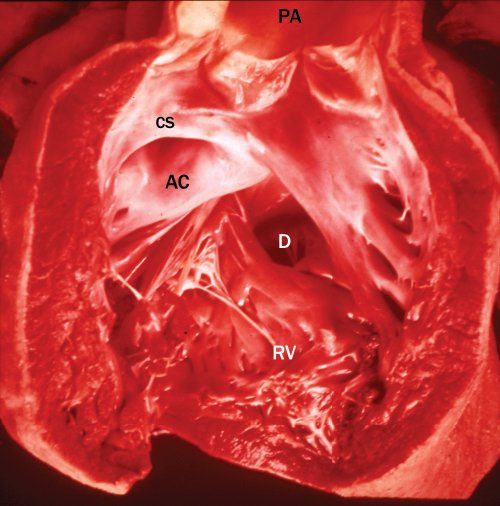
Figure 17.3. Pathologic specimen of double-outlet right ventricle. The right ventricle (RV) has been opened, demonstrating the origin of both great arteries from the RV. AC, aortic conus; CS, conus septum; D, defect (ventricular septal defect [VSD]); PA, pulmonary artery.
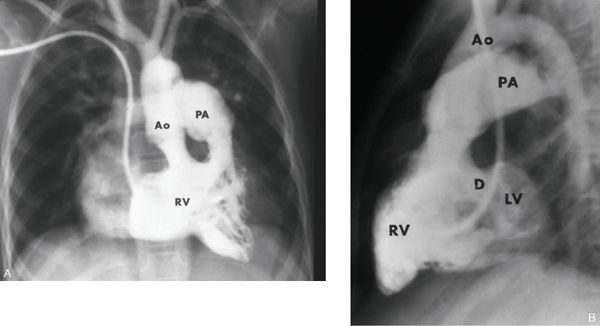
Figure 17.4. Right ventricular (RV) angiogram of double-outlet right ventricle. The frontal and lateral views illustrate features of DORV with subaortic VSD and side-by-side great arteries. Both semilunar valves are at the same horizontal plane and are side by side. In the lateral view, the VSD (D) is below the conus and allows some filling of the left ventricle (LV). Ao, aorta; PA, pulmonary artery. (With permission of the Mayo Foundation.)
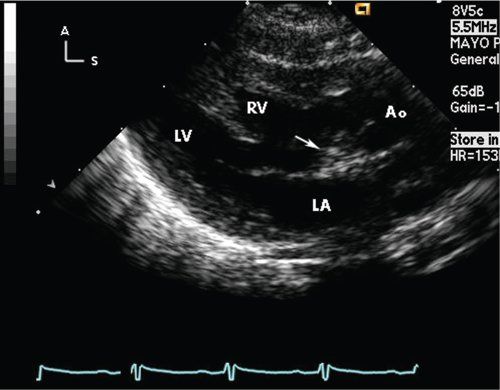
Figure 17.5. Parasternal long-axis scan in a neonate with double-outlet right ventricle, with side-by-side great arteries and subaortic ventricular septal defect (VSD). With the aorta (Ao) anterior and rightward, it is the only great artery observed in this scan. Arrow, subaortic conus separating the aortic valve from the mitral valve. LA, left atrium; LV, left ventricle; RV, right ventricle.
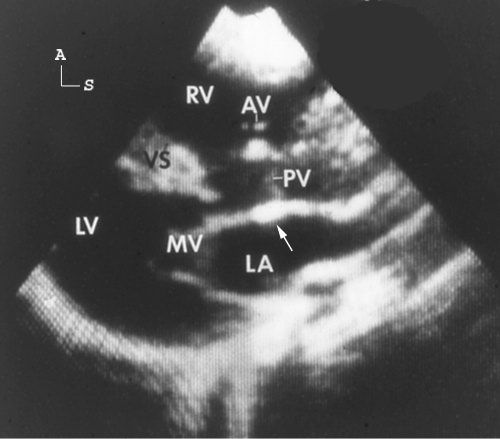
Figure 17.6. Parasternal long-axis scan in a patient with double-outlet right ventricle, with the aorta anterior and right of the pulmonary artery with a subpulmonary ventricular septal defect (VSD). There is a relatively small amount of conus tissue (arrow) separating the pulmonary valve (PV) from the mitral valve (MV). Both great arteries are entirely committed to the right ventricle (RV). LA, left atrium; LV, left ventricle.
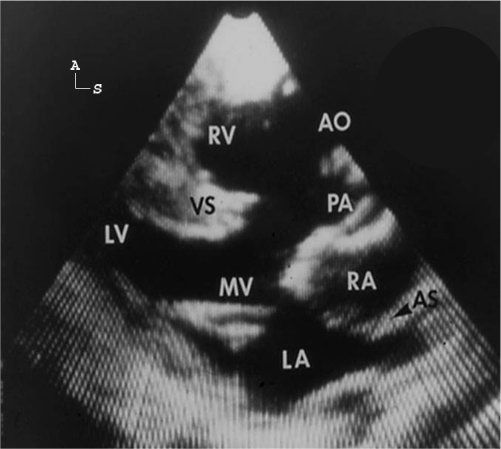
Figure 17.7. Parasternal long-axis scan in a patient with double-outlet right ventricle, with the aorta (Ao) anterior and to the left of the pulmonary artery (PA). Both great arteries are entirely committed to the right ventricular cavity and are observed in parallel orientation originating from the right ventricle (RV). In this standard long-axis scan, all four cardiac chambers and both great arteries are observed simultaneously. In this example, findings of left-juxtaposed atrial appendages are also evident. The right atrial (RA) appendage courses posterior to the great arteries to lie next to the left atrial appendage. The pulmonary valve appears slightly thickened and, in real time, the valve was dome shaped during systole, consistent with pulmonary stenosis. The mitral valve (MV) is markedly separated from the semilunar valves. AS, atrial septum (black arrow head); LA, left atrium; LV, left ventricle; TV, tricuspid valve; VS, ventricular septum. (Reprinted with permission from Hagler DJ, Tajik AJ, Seward JB, et al. Double-outlet right ventricle: wide-angle two-dimensional echocardiographic observations. Circulation. 1981;63:419–428.)
Parasternal long-axis scans are often obtained from a slightly more superior position at the left sternal edge. This view demonstrates the initial parallel course of the great arteries (Figs. 17.6, 17.7, and 17.10; Videos 17.1 to 17.6). The pulmonary artery may be recognized by its posterior course to the lungs and by its bifurcation into the right and left pulmonary arteries, as noted with short-axis (see Fig. 17.10) or subcostal scans. The more anterior and superior great artery is the aorta. Parasternal long-axis scans demonstrate mitral–semilunar valve discontinuity with the presence of muscular conus separation (see Figs. 17.5, 17.6, and 17.10). Mitral–semilunar valve discontinuity is demonstrated by two-dimensional echocardiography as a dense echo (fibromuscular) or muscular conus separating the two valves. As observed in Figure 17.4, the degree of separation is variable, but with high-resolution imaging (7- and 10-MHz transducers), it can be demonstrated even when 2 to 3 mm in size.
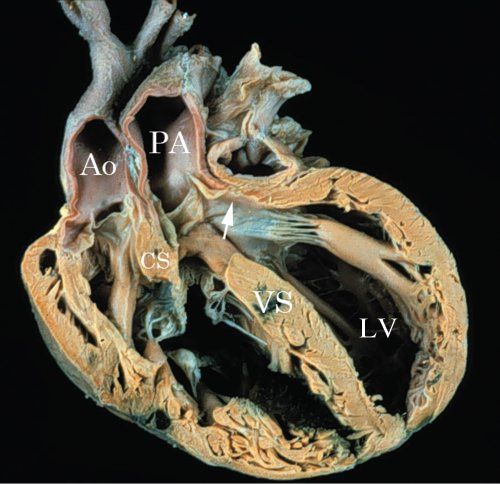
Figure 17.8. Pathologic specimen illustrating DORV with subpulmonary VSD. A sagittal section of DORV with subpulmonary VSD. The conus septum (CS) is malaligned with the lower ventricular septum (VS). The outlet from the left ventricle (LV) is directed into the pulmonary artery (PA). The aorta (Ao) is anterior and to the right.
Position of the Ventricular Septal Defect
Two-dimensional echocardiography accurately predicts the position of the VSD in reference to the great arteries. In most patients, typical subaortic or subpulmonary defects can be demonstrated by parasternal and subcostal scans (see Figs. 17.5, 17.6, 17.7, and 17.10). Doubly-committed defects appear nearly equally committed to both great arteries. Remote or noncommitted defects are isolated or multiple muscular VSDs or complete AV septal defects. Figure 17.11 illustrates a remote posterior muscular VSD in DORV. Neither great artery is committed to the VSD. Similarly, remote complete AV septal defects are best recognized on apical four-chamber or subcostal views (Fig. 17.12).
Doppler echocardiography and color flow Doppler may be helpful adjuncts for demonstrating these abnormalities as well as associated muscular VSDs. Continuous-wave Doppler interrogation of the VSD may demonstrate a high-velocity jet consistent with an LV-to-RV pressure gradient from a restrictive VSD. Although some associated muscular VSDs may be appreciated with color flow imaging, many centers recommend complete angiographic assessment if multiple muscular (“Swiss cheese septum”) VSDs are suspected.
DORV may be associated with a number of AV valve anomalies, including complete AV septal defect, isolated cleft of the anterior mitral leaflet, and overriding (atrial and ventricular septal malalignment) or straddling left or right AV valves. Apical and subcostal four-chamber views and short-axis scans easily demonstrate these AV valve abnormalities at the crux of the heart. It is particularly important to accurately delineate the location and points of insertion of the AV valve chordal apparatus. Abnormal chordal insertions of the tricuspid valve into the conus septum may prohibit surgical efforts to direct the left ventricular outflow anteriorly toward the aorta. Isolated clefts of the anterior mitral leaflet often have chordal attachments to the ventricular septum or attachments that straddle the ventricular septum into the RV. Figure 17.13 illustrates a pathologic specimen of DORV with subpulmonary VSD and straddling mitral valve. Associated anomalies with DORV include left-juxtaposed atrial appendage (see Fig. 17.7), ASD, anomalous systemic (left SVC to coronary sinus) and pulmonary venous connections, and coarctation of the aorta. Multiple imaging planes must be used to exclude these associated anomalies.
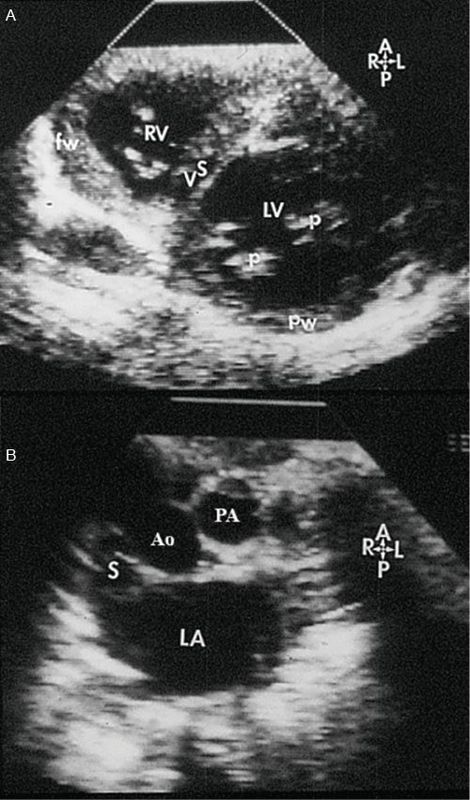
Figure 17.9. Short-axis scans of the heart from apex (top) to base (bottom) in a patient with double-outlet right ventricle. A: Short-axis scan at the mid-ventricular level. This image illustrates the plane of the ventricular septum (VS) below the level of the ventricular septal defect (VSD). B: Short-axis scan at the level of the great arteries. The great arteries are related normally. The superior vena cava (S) is to the right of the aorta (Ao). The pulmonary artery (PA) is to the left and anterior. Thus, the VSD is noted to be subaortic at the cardiac base. (Reprinted with permission from Hagler DJ, Tajik AJ, Seward JB, et al. Double-outlet right ventricle: wide-angle two-dimensional echocardiographic observations. Circulation. 1981;63:419–428.)
CORONARY ANOMALIES
Coronary artery patterns in DORV predominately are of three types: normal; abnormal, as in tetralogy of Fallot; and abnormal, as in transposition of the great arteries. The tetralogy of Fallot-like anomalies may have anomalous origin of the left anterior descending coronary artery from the right coronary artery. The pattern observed in transposition of the great arteries would have origin of the right coronary artery from the right posterior aortic cusp and origin of the left coronary artery from the left posterior cusp. They should be carefully assessed as described previously in these entities.
PHYSIOLOGY
The physiology observed in patients with DORV depends on the four positions of the VSD and the great artery relationships. It is also affected by other associated conditions, most notably PS, the presence of pulmonary vascular obstructive disease, ASD, and the size of the VSD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


