DISEASES OF THE AORTA
The aorta is the conduit through which blood ejected from the left ventricle is delivered to the systemic arterial bed. In adults, its diameter is approximately 3 cm at the origin and in the ascending portion, 2.5 cm in the descending portion in the thorax, and 1.8–2 cm in the abdomen. The aortic wall consists of a thin intima composed of endothelium, subendothelial connective tissue, and an internal elastic lamina; a thick tunica media composed of smooth-muscle cells and extracellular matrix; and an adventitia composed primarily of connective tissue enclosing the vasa vasorum and nervi vascularis. In addition to the conduit function of the aorta, its viscoelastic and compliant properties serve a buffering function. The aorta is distended during systole to allow a portion of the stroke volume and elastic energy to be stored, and it recoils during diastole so that blood continues to flow to the periphery. Because of its continuous exposure to high pulsatile pressure and shear stress, the aorta is particularly prone to injury and disease resulting from mechanical trauma. The aorta is also more prone to rupture than is any other vessel, especially with the development of aneurysmal dilation, since its wall tension, as governed by Laplace’s law (i.e., proportional to the product of pressure and radius), will be increased.
CONGENITAL ANOMALIES OF THE AORTA
Congenital anomalies of the aorta usually involve the aortic arch and its branches. Symptoms such as dysphagia, stridor, and cough may occur if an anomaly causes a ring around or otherwise compresses the esophagus or trachea. Anomalies associated with symptoms include double aortic arch, origin of the right subclavian artery distal to the left subclavian artery, and right-sided aortic arch with an aberrant left subclavian artery. A Kommerell’s diverticulum is an anatomic remnant of a right aortic arch. Most congenital anomalies of the aorta do not cause symptoms and are detected during catheter-based procedures. The diagnosis of suspected congenital anomalies of the aorta typically is confirmed by computed tomographic (CT) or magnetic resonance (MR) angiography. Surgery is used to treat symptomatic anomalies.
AORTIC ANEURYSM
An aneurysm is defined as a pathologic dilation of a segment of a blood vessel. A true aneurysm involves all three layers of the vessel wall and is distinguished from a pseudoaneurysm, in which the intimal and medial layers are disrupted and the dilated segment of the aorta is lined by adventitia only and, at times, by perivascular clot. Aneurysms also may be classified according to their gross appearance. A fusiform aneurysm affects the entire circumference of a segment of the vessel, resulting in a diffusely dilated artery. In contrast, a saccular aneurysm involves only a portion of the circumference, resulting in an outpouching of the vessel wall. Aortic aneurysms also are classified according to location, i.e., abdominal versus thoracic. Aneurysms of the descending thoracic aorta are usually contiguous with infradiaphragmatic aneurysms and are referred to as thoracoabdominal aortic aneurysms.
ETIOLOGY
Aortic aneurysms result from conditions that cause degradation or abnormal production of the structural components of the aortic wall: elastin and collagen. The causes of aortic aneurysms may be broadly categorized as degenerative diseases, inherited or developmental diseases, infections, vasculitis, and trauma (Table 38-1). Inflammation, proteolysis, and biomechanical wall stress contribute to the degenerative processes that characterize most aneurysms of the abdominal and descending thoracic aorta. These are mediated by B-cell and T-cell lymphocytes, macrophages, inflammatory cytokines, and matrix metalloproteinases that degrade elastin and collagen and alter the tensile strength and ability of the aorta to accommodate pulsatile stretch. The associated histopathology demonstrates destruction of elastin and collagen, decreased vascular smooth muscle, in-growth of new blood vessels, and inflammation. Factors associated with degenerative aortic aneurysms include aging, cigarette smoking, hypercholesterolemia, male sex, and a family history of aortic aneurysms.
TABLE 38-1
DISEASES OF THE AORTA: ETIOLOGY AND ASSOCIATED FACTORS

The most common pathologic condition associated with degenerative aortic aneurysms is atherosclerosis. Many patients with aortic aneurysms have coexisting risk factors for atherosclerosis (Chap. 30), as well as atherosclerosis in other blood vessels.
Cystic medial necrosis is the histopathologic term used to describe the degeneration of collagen and elastic fibers in the tunica media of the aorta as well as the loss of medial cells that are replaced by multiple clefts of mucoid material. Cystic medial necrosis characteristically affects the proximal aorta, results in circumferential weakness and dilation, and leads to the development of fusiform aneurysms involving the ascending aorta and the sinuses of Valsalva. This condition is particularly prevalent in patients with Marfan syndrome, Loeys-Dietz syndrome, Ehlers-Danlos syndrome type IV, hypertension, congenital bicuspid aortic valves, and familial thoracic aortic aneurysm syndromes; sometimes it appears as an isolated condition in patients without any other apparent disease.
Familial clusterings of aortic aneurysms occur in 20% of patients, suggesting a hereditary basis for the disease. Mutations of the gene that encodes fibrillin-1 are present in patients with Marfan syndrome. Fibrillin-1 is an important component of extracellular micofibrils, which support the architecture of elastic fibers and other connective tissue. Deficiency of fibrillin-1 in the extracellular matrix leads to excessive signaling by transforming growth factor β (TGF-β). Loeys-Dietz syndrome is caused by mutations in the genes that encode TGF-β receptors 1 (TGFBR1) and 2 (TGFBR2). Increased signaling by TGF-β and mutations of TGFBR1 and TGFBR2 may cause thoracic aortic aneurysms. Mutations of type III procollagen have been implicated in Ehlers-Danlos type IV syndrome. Linkage analysis has identified loci on chromosomes 5q13–14, 11q23.3–q24, and 3p24–25 in several families, although the specific alleles have not been described.
The infectious causes of aortic aneurysms include syphilis, tuberculosis, and other bacterial infections. Syphilis is a relatively uncommon cause of aortic aneurysm. Syphilitic periaortitis and mesoaortitis damage elastic fibers, resulting in thickening and weakening of the aortic wall. Approximately 90% of syphilitic aneurysms are located in the ascending aorta or aortic arch. Tuberculous aneurysms typically affect the thoracic aorta and result from direct extension of infection from hilar lymph nodes or contiguous abscesses as well as from bacterial seeding. Loss of aortic wall elasticity results from granulomatous destruction of the medial layer. A mycotic aneurysm is a rare condition that develops as a result of staphylococcal, streptococcal, Salmonella, or other bacterial or fungal infections of the aorta, usually at an atherosclerotic plaque. These aneurysms are usually saccular. Blood cultures are often positive and reveal the nature of the infective agent.
Vasculitides associated with aortic aneurysm include Takayasu’s arteritis and giant cell arteritis, which may cause aneurysms of the aortic arch and descending thoracic aorta. Spondyloarthropathies such as ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, relapsing polychondritis, and reactive arthritis (formerly known as Reiter’s syndrome) are associated with dilation of the ascending aorta. Aortic aneurysms occur in patients with Behçet’s syndrome and Cogan’s syndrome. Aortic aneurysms also result from idiopathic aortitis. Traumatic aneurysms may occur after penetrating or nonpenetrating chest trauma and most commonly affect the descending thoracic aorta just beyond the site of insertion of the ligamentum arteriosum. Chronic aortic dissections are associated with weakening of the aortic wall that may lead to the development of aneurysmal dilatation.
THORACIC AORTIC ANEURYSMS
The clinical manifestations and natural history of thoracic aortic aneurysms depend on their location. Cystic medial necrosis is the most common pathology associated with ascending aortic aneurysms, whereas atherosclerosis is the condition most frequently associated with aneurysms of the aortic arch and descending thoracic aorta. The average growth rate of thoracic aneurysms is 0.1–0.2 cm per year. Thoracic aortic aneurysms associated with Marfan syndrome or aortic dissection may expand at a greater rate. The risk of rupture is related to the size of the aneurysm and the presence of symptoms, ranging approximately from 2–3% per year for thoracic aortic aneurysms <4.0 cm in diameter to 7% per year for those >6 cm in diameter. Most thoracic aortic aneurysms are asymptomatic; however, compression or erosion of adjacent tissue by aneurysms may cause symptoms such as chest pain, shortness of breath, cough, hoarseness, and dysphagia. Aneurysmal dilation of the ascending aorta may cause congestive heart failure as a consequence of aortic regurgitation, and compression of the superior vena cava may produce congestion of the head, neck, and upper extremities.
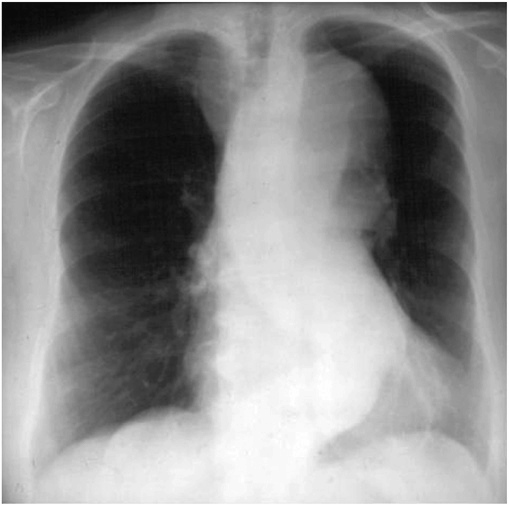
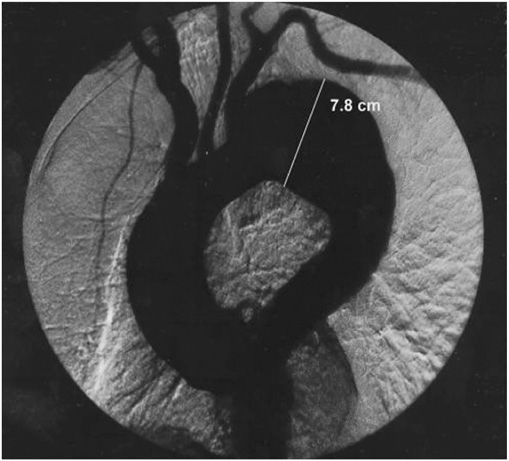
A chest x-ray may be the first test that suggests the diagnosis of a thoracic aortic aneurysm (Fig. 38-1). Findings include widening of the mediastinal shadow and displacement or compression of the trachea or left main-stem bronchus. Echocardiography, particularly transesophageal echocardiography, can be used to assess the proximal ascending aorta and descending thoracic aorta. Contrast-enhanced CT, magnetic resonance imaging (MRI), and conventional invasive aortography are sensitive and specific tests for assessment of aneurysms of the thoracic aorta and involvement of branch vessels (Fig. 38-2). In asymptomatic patients whose aneurysms are too small to justify surgery, noninvasive testing with either contrast-enhanced CT or MRI should be performed at least every 6–12 months to monitor expansion.
FIGURE 38-1
A chest x-ray of a patient with a thoracic aortic aneurysm.
FIGURE 38-2
An aortogram demonstrating a large fusiform aneurysm of the descending thoracic aorta.
TREATMENT Thoracic Aortic Aneurysms
β-Adrenergic blockers currently are recommended for patients with thoracic aortic aneurysms, particularly those with Marfan syndrome, who have evidence of aortic root dilatation to reduce the rate of further expansion. Additional medical therapy should be given as necessary to control hypertension. Recent preliminary studies indicate that angiotensin receptor antagonists and angiotensin-converting enzyme inhibitors will reduce the rate of aortic dilation in patients with Marfan syndrome by blocking TGF-β signaling; clinical outcome trials of this treatment approach are in progress. Operative repair with placement of a prosthetic graft is indicated in patients with symptomatic thoracic aortic aneurysms, those in whom the ascending aortic diameter is >5.5–6 cm or the descending thoracic aortic diameter is >6.5–7 cm, and those with an aneurysm that has increased by >1 cm per year. In patients with Marfan syndrome or bicuspid aortic valve, ascending thoracic aortic aneurysms >5 cm should be considered for surgery. Endovascular repair is an alternative treatment for some patients with descending thoracic aortic aneurysms.
ABDOMINAL AORTIC ANEURYSMS
Abdominal aortic aneurysms occur more frequently in males than in females, and the incidence increases with age. Abdominal aortic aneurysms ≥4.0 cm may affect 1–2% of men older than 50 years. At least 90% of all abdominal aortic aneurysms >4.0 cm are related to atherosclerotic disease, and most of these aneurysms are below the level of the renal arteries. Prognosis is related to both the size of the aneurysm and the severity of coexisting coronary artery and cerebrovascular disease. The risk of rupture increases with the size of the aneurysm: the 5-year risk for aneurysms <5 cm is 1–2%, whereas it is 20–40% for aneurysms >5 cm in diameter. The formation of mural thrombi within aneurysms may predispose to peripheral embolization.
An abdominal aortic aneurysm commonly produces no symptoms. It usually is detected on routine examination as a palpable, pulsatile, expansile, and nontender mass, or it is an incidental finding observed on an abdominal x-ray or ultrasound study performed for other reasons. As abdominal aortic aneurysms expand, however, they may become painful. Some patients complain of strong pulsations in the abdomen; others experience pain in the chest, lower back, or scrotum. Aneurysmal pain is usually a harbinger of rupture and represents a medical emergency. More often, acute rupture occurs without any prior warning, and this complication is always life threatening. Rarely, there is leakage of the aneurysm with severe pain and tenderness. Acute pain and hypotension occur with rupture of the aneurysm, which requires an emergency operation.
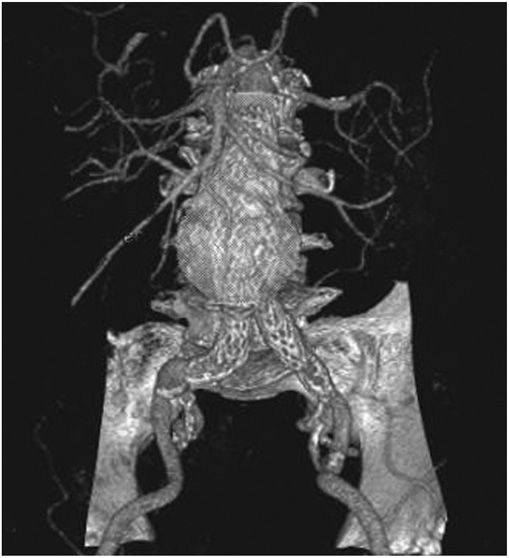
Abdominal radiography may demonstrate the calcified outline of the aneurysm; however, about 25% of aneurysms are not calcified and cannot be visualized by x-ray imaging. An abdominal ultrasound can delineate the transverse and longitudinal dimensions of an abdominal aortic aneurysm and may detect mural thrombus. Abdominal ultrasound is useful for serial documentation of aneurysm size and can be used to screen patients at risk for developing an aortic aneurysm. In one large study, ultrasound screening of men age 65–74 years was associated with a risk reduction in aneurysm-related death of 42%. For this reason, screening by ultrasonography is recommended for men age 65–75 years who have ever smoked. In addition, siblings or offspring of persons with abdominal aortic aneurysms, as well as individuals with thoracic aortic or peripheral arterial aneurysms, should be considered for screening for abdominal aortic aneurysms. CT with contrast and MRI are accurate noninvasive tests to determine the location and size of abdominal aortic aneurysms and to plan endovascular or open surgical repair (Fig. 38-3). Contrast aortography may be used for the evaluation of patients with aneurysms, but the procedure carries a small risk of complications such as bleeding, allergic reactions, and atheroembolism. Since the presence of mural thrombi may reduce the luminal size, aortography may underestimate the diameter of an aneurysm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree