Diseases due to Nontuberculous Mycobacteria
Nontuberculous mycobacteria (NTM) are ubiquitous in our environment so that exposure to these organisms is universal and unavoidable.1 Unlike traditional tuberculosis, most of the approximately 150 currently identified NTM species are either nonvirulent or have low virulence for humans. The prevalence of NTM is rising in the United States, with many epidemiologic reports suggesting that NTM lung disease has surpassed tuberculosis.2,3 Clinicians are encountering NTM infections with increasing frequency and a general knowledge of common pathogens and diagnostic criteria are essential for the care and successful treatment of these individuals.
Prior to the AIDS epidemic, most NTM cases presented as indolent, cavitating pulmonary infections in persons with underlying lung diseases such as chronic obstructive pulmonary disease (COPD) or previous tuberculosis. During the 1980s, infections due to the more common NTM (Mycobacterium avium, some M. kansasii, some unclassified) emerged as complications of acquired immunodeficiency syndrome (AIDS). M. avium and M. intracellulare were termed M. avium complex or MAC infections.4,5 Subsequently, a syndrome of predominantly mid–lung zone bronchiectasis with MAC pulmonary infection was described in otherwise healthy middle-aged women, many with distinct body morphotypes including scoliosis, pectus excavatum, and mitral valve prolapse.6,7 This syndrome is often referred to as Lady Windermere syndrome.8 Mycobacterial infections after solid-organ and hematopoietic transplantation have also increased in frequency, reflecting both increased exposure and/or improved diagnostic methods, and almost universal use of central venous access devices.9 In the absence of mandatory infection reporting, the true incidence of positive cultures or NTM disease in the general population and transplant recipients in the United States can only be estimated.
ORGANISMS AND DEVELOPMENT OF DISEASE
The microbiology, epidemiology, and pathogenesis of nontuberculous mycobacterial infections are considered below.
 MICROBIOLOGY
MICROBIOLOGY
Almost 150 NTM species have now been identified and speciated. The increased number of species reflects improved microbiologic techniques for isolating NTM from clinical specimens and the use of 16 S rRNA gene sequencing as a standard for defining new species.10,11 To simplify understanding of these organisms, particularly as applied to clinical circumstances, they often are grouped into complexes of closely related species. The M. avium complex, for example, consists of multiple species, with the most frequent being M. avium and M. intracellulare. Certain species, such as M. kansasii are associated with human disease more often than others and are presumed to be more virulent while species such as M. gordonae, M. terrae complex, and M. mucogenicum most often represent contamination of the respiratory tract from exposure to tap water.12
A number of important considerations in the laboratory evaluation should be recognized:
1. Clinicians must be aware that these organisms may be present and request mycobacterial cultures in appropriate specimens. The Gram stain will not adequately detect mycobacteria. The preferred staining procedure is the fluorochrome method, although the Ziehl–Neelsen method and Kinyoun stain are less sensitive alternatives. NTM are often more sensitive to the acid-fast decolorization procedure.
2. Some specific guidelines have been adopted to avoid potential sources of contamination, especially tap water, in specimen collection. Mouth rinsing or brushing of teeth before collection should not be done. Specimens should be submitted without fixatives. Refrigeration of samples at 4°C should be used if transportation to the laboratory is delayed for over 1 hour.
3. Specimens should be cultured using both liquid and solid media. Species that are often associated with cutaneous and lymph node disease may require special growth conditions and/or lower incubation temperatures (28–32°C), including M. haemophilum, M. genavense, M. marinum and the rapidly growing mycobacteria (RGM). Methods for the isolation of NTM in clinical laboratories have been approved by the Clinical and Laboratory Standards Institute (CLSI).13
4. Molecular testing is used primarily (initially) to distinguish M. tuberculosis from NTM species. DNA probes exist for the most commonly encountered slowly-growing NTM species (M. avium complex or MAC, M. avium, M. intracellulare, M. kansasii, and M. gordonae). There are no probes for rapidly growing species such as M. fortuitum or M. abscessus.
5. NTM should be identified to the species level.
6. American Thoracic Society (ATS) guidelines and CLSI recommend that routine susceptibility testing be limited for MAC isolates to the macrolides (clarithromycin) and for M. kansasii isolates rifampin and clarithromycin.12,13 For less frequently isolated, slowly growing mycobacteria such as M. simiae, M. xenopoi, etc. more extensive testing is recommended.13
7. For rapidly growing mycobacteria, in particular M. fortuitum, M. abscessus, M. chelonae, isolates should be tested against a more extended array of antibiotics including amikacin, imipenem, and clarithromycin.
Respiratory Specimens
To establish the diagnosis of NTM lung disease, sputum is often induced with 7% hypertonic saline or collected via bronchoscopy (Table 132-1). Alternatively, three expectorated specimens may be collected on different days. Respiratory specimens can be shipped to the laboratory by mail using standard shipping guidelines for laboratory samples. Lung biopsy or other tissue (e.g., lymph node) biopsy specimens can also be used for cultures.
TABLE 132-1 Clinical and Microbiologic Criteria for Diagnosing Nontuberculous Mycobacterial (NTM) Lung Disease
Source: Adapted with permission from Griffith DE, Aksamit T, Brown-Elliott BA, et al. An Official ATS/IDSA Statement: diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416.
 EPIDEMIOLOGY AND PATHOGENESIS
EPIDEMIOLOGY AND PATHOGENESIS
NTM are ubiquitous in the environment, often isolated from soil and water, including household water sources. There is increasing concern that biofilms that form in municipal and household water supplies may be a significant harbor of NTM.14–16 M. avium, M. kansasii, M. simiae, and M. xenopi are readily recovered from tap water in areas where these species are common. There has been no documentation of animal-to-human and rarely human-to-human transmission of NTM, including highly susceptible hosts (e.g., those with cystic fibrosis).17–23
NTM may cause both asymptomatic infection and symptomatic disease in humans. NTM are often detected in asymptomatic patients awaiting organ transplantation with diffuse nodular or interstitial lung disease. Skin test studies in adults indicate that a substantial proportion have had prior infection with NTM, notably in the southeastern United States.24 While disease is often asymptomatic (and often in individuals with other underlying lung disease), in the immunologically normal host, NTM has not been shown to lead to latent infection. However, disease may be exacerbated by immune suppression.
The most common clinical manifestation of NTM infection is lung disease, but lymphatic, skin/soft tissue, and disseminated disease are also encountered.25,26 CDC reports that of NTM isolates reported to the Public Health Laboratory System (PHLIS) database between 1993 and 1996, 75% were pulmonary, 5% were from blood, 2% from skin/soft tissue, and 0.4% from lymph nodes.3 Disseminated NTM, primarily M. avium, infections occurs in HIV-infected patients with CD4+ T-lymphocyte counts below 50/cc.27,28
In individuals with congenital immune deficiencies such as chronic granulomatous disease, there may be defects in production of interferon (IFN)-γ or interleukin (IL)-12, or defects in receptors or pathways controlling responses to IFN-γ.29,30 These IFN-γ pathway defects include receptor and signaling mutations in the nuclear factor-κB essential modulator, IFN-γ receptor 1 and receptor 2, IL-12 receptor 1 subunit, IL-12 subunit p40, and the signal transducer and activator of transcription 1 (STAT1). These defects are important given the role of macrophages in the innate immune response to NTM with IL-12 and IFN-γ essential for intracellular killing of mycobacteria. These patients, however, represent a small minority of patients with NTM disease.
Inhibitors of tumor necrosis factor (TNF-α) suggests that TNF also is essential in the prevention of disease activation.31–34 These agents have been used in a variety of chronic inflammatory conditions, including inflammatory bowel disease and rheumatoid arthritis. The effect of these agents may persist for months to years after administration. In addition to NTM infection, such agents have been associated with activation of Aspergillus species, histoplasmosis, coccidioidomycosis listeriosis, and especially M. tuberculosis.33,34
Pulmonary Disease
There are two distinct prototypes of individuals with pulmonary NTM disease including nonsmoking postmenopausal women with nodular bronchiectasis, primarily involving the right middle lobe and the lingula (the Lady Windermere disease) and individuals with a long history of tobacco use and severe chronic obstructive lung disease (COPD) who present with fibrocavitary upper lobe changes. Women without obvious immune defects who have developed pulmonary NTM disease often have distinct body characteristics that may include scoliosis, low body mass index, pectus excavatum, mitral valve prolapse, and joint hypermobility.6,7 The relationship between these physical traits and NTM infection remains elusive. Cavitary lung disease associated with COPD patients present formidable challenges to the clinician as treatment generally requires prolonged IV therapy as well as consideration of adjunctive surgery (Table 132-2). Disease (especially upper lobe fibrocavitary disease) should be treated as caused by M. tuberculosis until identification of the organism proves otherwise.
aPeak levels should be monitored at least weekly with a goal of 20–25μg/mL.
CLINICAL PRESENTATION
Most patients have pre-existing structural lung disease (Figs. 132-1 and 132-2) and present with chronic cough or sputum production, often with nonspecific signs, including dyspnea, fatigue, malaise, low-grade fevers, and weight loss. Both types of lung disease may present with hemoptysis. Physical examination is generally unrewarding, with auscultation of lungs ranging from diffuse rhonchi and wheezes to inspiratory squeaks. As noted, some women have a characteristic morphotype and are frequently otherwise in very good health.
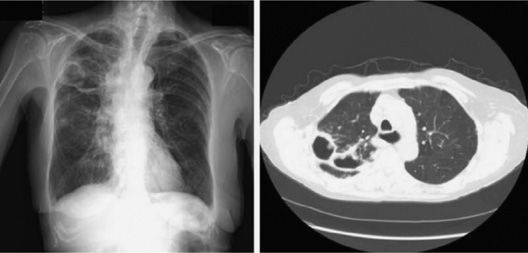
Figure 132-1 Right upper lobe cavitary MAC disease in a patient with COPD. (A) Chest radiograph. (B) CT scan.
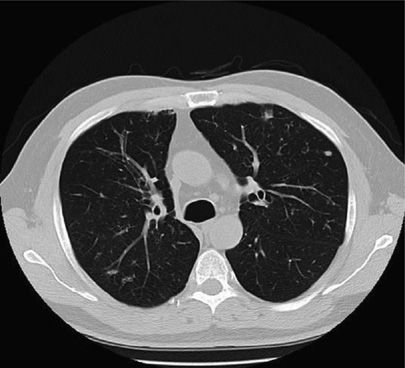
Figure 132-2 Chest CT scan showing bronchiectasis and nodular opacities in an 86-year-old female with MAC.
Three forms of disease merit comment: hypersensitivity pneumonitis (HP) with NTM infection, infection in cystic fibrosis, and infection in transplant recipients. The hypersensitivity lung disease has been termed “hot tub lung.” This unique form of HP is primarily seen after exposure to NTM from indoor hot tubs and spas, and occasionally contaminated showers, with aerosolization devices.35–38 The hot tubs are often poorly maintained and the water contain large numbers of NTM (almost all M. avium). Treatment predominately includes exposure avoidance with the use of prednisone and/or antimicrobial agents.12
A similar HP syndrome is seen in metal grinders (auto/machine workers, etc.) associated with exposures to metalworking fluids (paraffins, pine oils, and polycyclic aromatic hydrocarbons) containing mycobacteria.39,40 This syndrome is associated almost exclusively with M. immunogenum, a rapidly growing mycobacterium. Onset of symptoms is subacute and is generally in nonsmokers without a clear disposition to infection. Chest radiographs and chest CT scans have diffuse small nodules with ground-glass infiltrates. Lung biopsy generally reveals centrilobular and bronchocentric nonnecrotizing granulomata, although some cases may have necrotizing granulomas, bronchiolitis and organizing pneumonia, or interstitial inflammation.
In cystic fibrosis, NTM are being increasingly isolated, often with uncertain clinical significance. As many as 20% of adolescent cystic fibrosis individuals, especially in the south in areas of Florida, Texas, and Louisiana, have at least one culture positive for NTM.12,19 As a result, all routine sputum samples obtained in these patients should be cultured for both routine pathogens such as Pseudomonas aeruginosa and for NTM. This is especially important in patients who are given long-term azithromycin (a macrolide) for its anti-inflammatory properties. M. abscessus is the most common NTM in this setting and is increasingly being treated as a pathogen.
In solid-organ transplant recipients, NTM disease has been historically difficult to define with the most common manifestations of infection including cutaneous and pleuropulmonary disease (see Chapter 123).9,41–43 Catheter-related infection is the most commonly reported manifestation of NTM disease in hematopoietic stem-cell transplant recipients.9 In such hosts, skin and pulmonary lesions should be biopsied for histology, special staining, and microbiologic cultures, including bacteria, Nocardia species, fungi, and mycobacteria. NTM infections associated with catheters may be documented by tunnel or blood cultures. The RGM, especially M. fortuitum, are the most common pathogens. The pattern of NTM infection in transplantation differs from that of disseminated HIV-infection (more commonly M. avium complex), which limits extrapolation of therapeutic data from HIV-infected individuals to this population. Drug interactions are common and outlined in Table 132-3. Catheter-related infections have also been associated with a newly described pigmented RGM, M. bacteremicum.44
RADIOGRAPHY
Radiographic patterns associated with the aforementioned two phenotypes are uniquely distinct. The classic “Lady Windermere” pattern is associated with mid-lung field nodules and bronchiectasis, which may be accompanied by cavitations. On the opposite end of the spectrum, patients with COPD who have a long smoking history often present with large cavitary changes predominately located in the upper lobe lung zones, usually associated with a fibrotic pattern. There can be overlap of these disease spectrums making radiographic findings often diffuse and nonspecific.
TREATMENT
The approach to treatment varies with the species. Most species respond to regimens of several drugs. Toxicity and drug interactions are common (Tables 132-3 and 132-4). Therapy should be based on selected antimicrobial susceptibility testing that differs with each species or species complex. During therapy, monitoring for toxicity of drugs is essential. Such testing should include visual acuity, red–green color discrimination (ethambutol and rifabutin), liver enzymes (clarithromycin, azithromycin, rifabutin, rifampin, and isoniazid), auditory and vestibular function (streptomycin, amikacin, and azithromycin), renal function (streptomycin and amikacin), and leukocyte and platelet counts (rifampin, rifabutin, linezolid, trimethoprim–sulfamethoxazole and streptomycin). Clarithromycin enhances rifabutin toxicity (especially uveitis), whereas the rifamycin (rifampin more than rifabutin) is a potent inducer of the cytochrome p-450 system and can lower clarithromycin serum drug levels as well as steroids, anticoagulants, statins and β- blockers as a result of increased metabolism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree