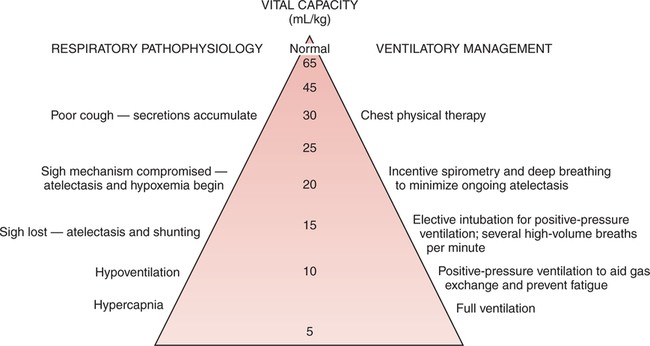
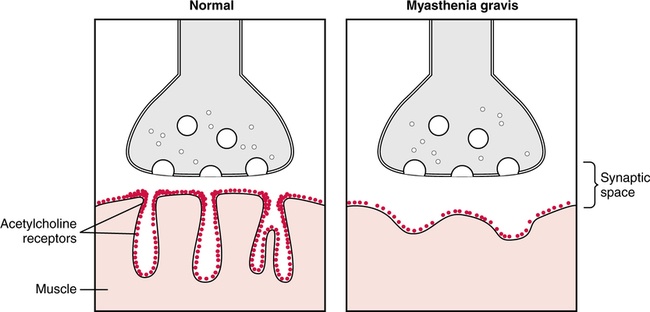
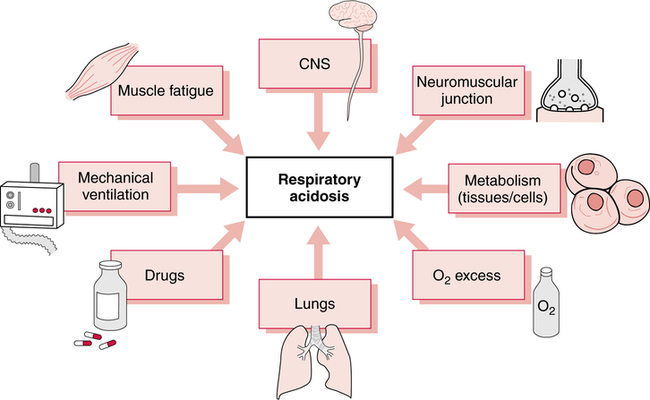
A systematic method for classification of acid-base status, based on the arterial blood gas report, was described in Chapter 2, and limits, rules, and steps in classification were clearly delineated. Application of these principles leads to consistent results regardless of the background of the interpreter. In fact, these limits and steps can be programmed into a computer that will provide reproducible classifications. The clinical manifestations of acute hypercapnia are predominantly neurologic.485 Symptoms may vary from anxiety and irritability to lethargy and somnolence. Pulmonary symptoms may include dyspnea and distress. Stupor and coma appear to be rare but may occur when PaCO2 exceeds 70 mm Hg.485 It is also important to remember that hypercapnia causes increased cerebral perfusion and intracranial pressure. Indeed, during acute hypercapnia, cerebral blood flow may more than double while, conversely, it will decrease by more than 50% when PaCO2falls by about 10 mm Hg.496 This may be especially important in the patient with CNS trauma or following CNS surgery where low intracranial pressure is a goal in patient treatment. In chronic hypercapnia, cerebral blood flow is normal and responsiveness to changes in PaCO2 is reduced. It appears that interstitial acidosis is really the primary regulator of cerebral dilation and perfusion.497 Cardiovascular effects in mild to moderate hypercapnia typically include an increased cardiac output and tachycardia mediated through an adrenergic response. On clinical examination, the skin may be flushed and warm. The patient may also be diaphoretic and demonstrate a bounding pulse. In severe hypercapnia, hypotension and/or arrhythmia may occur as a direct effect of PaCO2 on the myocardium and vasculature.485 Physiologic changes may be more related to concurrent acidemia and hypoxia than hypercapnia. Certainly there is a less dramatic response when hypercapnia develops slowly. The somewhat innocuous nature of hypercapnia has lead to the therapeutic approach of “permissive hypercapnia”498 where hypercapnia is tolerated in an effort to avoid excessive alveolar volume and pressure (see Chapter 10). Many believe that substantial hypercapnia is likely associated with very few adverse effects.498 As discussed previously, it is important to identify the underlying cause of the acid-base disorder to optimize management. Some common causes of respiratory acidosis are shown in Box 13-1. Some may find it easier to think of the potential origins of the various fundamental acid-base disturbances. For example, as shown in Figure 13-1, eight common sources of primary respiratory acidosis are: the lungs (e.g., chronic obstructive pulmonary disease [COPD]), drugs (e.g., anesthetics/narcotics), mechanical ven- tilation (e.g., iatrogenic hypoventilation), muscle fatigue (e.g., status asthmaticus), central nervous system (CNS; e.g., central alveo- lar hypoventilation), neuromuscular junction (e.g., Guillain-Barré syndrome), increased metabolism in cells/tissues (e.g., burn patients), and oxygen excess depressing ventilatory drive (e.g., in COPD). Chronic respiratory acidosis is only seen in approximately 25% to 33% of patients with significant chronic airflow obstruction.499 Chronic hypercapnia appears to be a mechanism whereby some patients avoid excessive respiratory muscle fatigue. Indeed, because of the hypercapnia, these patients can eliminate CO2 with far less ventilatory effort.499 500 Therefore, hypercapnia appears to be a useful strategy for avoiding inspiratory muscle overloading and failure.499 Notwithstanding, due to their limited ventilatory reserve, acute pneumonia can and frequently does lead to a superimposed acute ventilatory failure in these individuals.501 As described previously, chronic respiratory acidosis is common in end-stage COPD. In addition, when high concentrations of oxygen are administered to patients with end-stage COPD (especially those with hypercarbia), they may manifest an acute rise in PaCO2 levels above their chronically elevated baseline PaCO2. This acute respiratory acidosis is most apt to occur when PaCO2 levels are very high and/or when PaO2 levels are very low at the time when the oxygen is administered.486 487 Actually, baseline hypoxia and acidosis are better predictors of those patients likely to have worsening respiratory acidosis than baseline PaCO2.485 Even slight elevations in FIO2 may cause this effect.488 489 The acute hypercarbia may be progressive and may occasionally result in respiratory arrest. The rise in PaCO2 may be caused by obliteration of the hypoxic drive of the peripheral chemoreceptors; however, some evidence suggests that it is due primarily to a worsening of ventilation-perfusion matching.490 In all likelihood, it is a multi-factorial response. Regardless of the potential for acute respiratory acidosis, when hypoxia is suspected, oxygen must be administered in doses sufficient to relieve it. The target of oxygen therapy in COPD is typically a PaO2 of approximately 60 mm Hg, although not higher.486 491 When acute respiratory acidosis is observed in a patient with COPD who has a PaO2 level greater than 60 mm Hg, the FIO2 may be excessive. The higher the PaO2 and FIO2level, the more likely that the respiratory acidosis is at least in part related to the oxygen therapy. Interestingly, oxygen therapy may worsen hypercapnia in other chronic disorders as well. Although the mechanism is unclear, these include patients with neuromuscular disease, asthma, diaphragmatic dysfunction, or obesity hypoventilation syndrome.485 Depressant drugs such as morphine may diminish respiratory drive,492 with resultant hypercarbia and acidosis. The response of a given patient depends on the individual, the drug, and the dosage. Barbiturates, anesthetics, narcotics, and sedatives may cause this effect. Narcotic overdose characteristically manifests itself in a slow respiratory rate in the spontaneously breathing patient. Guillain-Barré syndrome is characterized by loss of reflexes and symmetric paralysis, typically beginning in the legs, with eventual nearly complete or complete recovery.502 Acute Guillain-Barré usually begins with fine paresthesias in the toes or fingertips, followed within days by leg weakness that makes walking and climbing stairs difficult. Weakness usually ascends and pain is common. Approximately two-thirds of cases follow an infection. Increased protein in the cerebrospinal fluid is a valuable diagnostic marker. Patients with very low (i.e., < 18 mL/kg) or rapidly declining vital capacities should be transferred and observed in ICU. The trend of neuromuscular dysfunction on ventilation can be observed at the bedside through serial measurements of vital capacity. A falling vital capacity may indicate progressive hypoventilation and perhaps the onset of respiratory acidosis. Figure 13-2 designates suggested clinical management of the Guillain-Barré syndrome based on progressive deterioration of the vital capacity. Myasthenia gravis affects approximately 25,000 people each year in the United States. The basic abnormality in myasthenia gravis is a decrease in the number of acetylcholine receptors in the neuromuscular junction.503 Figure 13-3 illustrates the decreased number of acetylcholine receptors and the widened synaptic space in myasthenia gravis as compared to the normal neuromuscular junction. The clinical result of this disease is neuromuscular weakness and fatigue. As described in Chapter 8 in the section on CO2 homeostasis, the PaCO2 depends not only on the quantity of CO2 leaving the blood (i.e., As described in Chapter 7, the respiratory quotient (RQ) relates CO2 production to oxygen consumption ( Total parenteral nutrition (TPN) is a nutritional support formula administered intravenously to critically ill patients to avoid the adverse effects of malnutrition.493 TPN consists of a mixture of glucose and amino acids. As such, TPN is high in carbohydrates and increases the RQ and the production of CO2 after administration. In the patient unable to meet the increased ventilatory requirement necessary to excrete this additional CO2, respiratory acidosis may ensue. Specifically, acute respiratory acidosis has been observed in patients with chronic lung disease in response to the administration of TPN.494 This effect may occur both in nonintubated patients and in patients on mechanical ventilation.494 495 During mechanical ventilation, the risk of TPN-induced respiratory acidosis is reduced if the minute volume of the ventilator is increased just before TPN administration.495 In addition, the development of hypercapnia has been reported in two young patients without COPD during weaning from mechanical ventilation while receiving TPN.338 Furthermore, when the number of carbohydrate calories given to these patients was decreased, CO2 production likewise dropped, and the respiratory acidosis was corrected.338 In summary, a high RQ may contribute to the onset or maintenance of respiratory acidosis. Malignant hyperthermia (MH) is an inherited condition in which some medications (especially anesthetics) trigger sustained skeletal muscle contraction and hypermetabolism.504 Symptoms include rapid, acute severe respi- ratory acidosis; hyperthermia; ventricular dysrhythmias; hyperkalemia; and muscular rigidity.505 The clinical course of the disorder is short, usually 1 to 3 days. Untreated, MH may have a 70% mortality.504 Treatment includes termination of the triggering agent, and control of pH, temperature, and potassium.505 Patients with severe burns also have an increase in total body metabolism secondary to tissue destruction and the reparative process. This response is greater than the hypermetabolism seen in sepsis or other forms of trauma. The magnitude and duration of the metabolic response parallel burn severity with metabolism doubling in a 60% total body burn.506 Other causes of hypermetabolism include sepsis, fever, thyrotoxicosis, and trauma.485 In fever, CO2 production will increase approximately 13% for each degree centigrade elevation in body temperature. TPN is associated not only with a high RQ; it also has a thermic effect secondary to the protein component of the solution.550 Consequently, TPN tends to increase CO2 production through changes in both the type and quantity of metabolism.550 Indeed the thermic effect appears to have an even stronger impact on CO2 production than the type of substrate used for metabolism. The administration of excess calories will also lead to increased CO2 production through lipogenesis, which has an RQ of nearly 8.0.507 Thus, the number of calories, the percentage of carbohydrate, and the nature of the patient’s illness must all be considered regarding the CO2 production load. In contrast, CO2 production may sometimes be reduced by decreased glucose intake, cooling, or paralyzing the patient.498 Administration of sodium bicarbonate (NaHCO3) intravenously also increases blood CO2 levels via the hydrolysis reaction. In spontaneously breathing individuals who are capable of increasing alveolar ventilation, this excess CO2 is immediately excreted. However, in the patient unable to excrete the additional blood CO2 (e.g., because of neurologic disease or controlled mechanical ventilation), hypercarbia and acute respiratory acidosis develop.365 Severe hypercapnia and respiratory acidosis of mixed venous blood has been shown to accompany resuscitation during cardiac arrest.567 It is presumed that these mixed venous gases reflect tissue conditions. The administration of NaHCO3 in this setting may further elevate the tissue PCO2 and thus exacerbate the tissue acidosis. This issue is discussed in greater detail in Chapter 14 in the section on treatment of metabolic acidosis. Patients with respiratory alkalosis often present with dyspnea and chest pain or tightness.512 In addition to renal compensation (i.e., decreased [HCO3]) for respiratory alkalosis, hypocapnia will decrease cerebral blood flow, alter some electrolyte concentrations, and increase the production of lactic acid. Regarding potassium, there is an initial abrupt onset of hyperkalemia. This is rapidly followed by the development of hypokalemia.508 Typically, serum potassium will decrease 0.3 mEq/L for each 0.1 unit increase in pH.508 Occasionally, electrolyte disturbances will be associated with muscle spasm. In addition, respiratory alkalosis increases production and decreases the clearance of lactic acid; however, the increase in lactic acid levels is only modest.508 In patients admitted to the emergency department, one hospital found the most common presenting complaints with substantial hypocapnia were dyspnea (61%), chest pain or tightness (43%), paresthesias (35%), panic (30%), dizziness (13%), palpitations (13%), and muscle spasm (9%).512 Finally, acute respiratory alkalosis may be associated with nausea, vomiting, or changes in gastrointestinal motility.508 Although mild hypocapnia may be seen in females and children younger than the age of 3 years, most often respiratory alkalosis is not normal. Some of the most common causes of respiratory alkalosis are shown in Box 13-2. Six possible origins of primary respiratory alkalosis are shown in Figure 13-4. These include the lungs (e.g., pulmonary fibrosis), drugs (e.g., salicylate toxicity), mechanical ventilation, the CNS (e.g., tumor), the cardiovascular system (e.g. cardiogenic shock), and thoracic cage abnormalities (e.g., scoliosis).
Differential Diagnosis of Acid-Base Disturbances
INTRODUCTION
RESPIRATORY ACIDOSIS
Physiologic Response to Respiratory Acidosis (Hypercapnia)
Common Causes of Respiratory Acidosis
Chronic Obstructive Pulmonary Disease
Oxygen Excess in Chronic Obstructive Pulmonary Disease
Drugs
Neuromuscular Disorders
Neuromuscular Disease
Excessive CO2 Production
 A), but also on metabolism and CO2 production (
A), but also on metabolism and CO2 production ( CO2). The significance and effects of CO2 production on ventilation and acid-base status in critically ill patients have only recently been appreciated. Carbon dioxide production depends on both the type and the quantity of metabolism.
CO2). The significance and effects of CO2 production on ventilation and acid-base status in critically ill patients have only recently been appreciated. Carbon dioxide production depends on both the type and the quantity of metabolism.
Type of Metabolism
The Respiratory Quotient and CO2 Production.
 CO2/
CO2/ O2). The numeric value of the RQ, in turn, depends on the type of body fuel being metabolized. Fat metabolism for example, results in less CO2 production (RQ of 0.7) than carbohydrate metabolism (RQ of 1.0).
O2). The numeric value of the RQ, in turn, depends on the type of body fuel being metabolized. Fat metabolism for example, results in less CO2 production (RQ of 0.7) than carbohydrate metabolism (RQ of 1.0).
Total Parenteral Nutrition and the Respiratory Quotient.
Quantity of Metabolism: Thermic Effect
Sodium Bicarbonate Administration
RESPIRATORY ALKALOSIS
Physiologic Response to Respiratory Alkalosis (Hypocapnia)
Overall Appearance
Common Causes of Respiratory Alkalosis
Differential Diagnosis of Acid-Base Disturbances