Differential Diagnosis and Evaluation of Sleepiness
Excessive daytime sleepiness (EDS) is a common problem affecting large segments of the general population. Although estimates depend on how sleepiness is defined (i.e., sleeping too much vs. falling asleep in the daytime), about 16% of adults experience sleepiness that affects their daytime function, and there is increasing evidence that sleepiness plays a part in both industrial and road traffic accidents. The National Highway Traffic Safety Administration estimates that more than 100,000 automotive crashes per year are fatigue related. These sleepiness-related accidents contribute to 40,000 injuries and 1550 deaths per year.1 Over the past two decades, research has provided increased understanding of obstructive sleep apnea (OSA), among other sleep disorders.2
The current prevalence estimates of moderate to severe sleep-disordered breathing with an apnea–hypopnea index, measured as events/h, ≥15 are thought to be higher, with contributing factors such as obesity increasing the prevalence. These estimated prevalence rates represent substantial increases over the last 20 years (relative increases of between 14% and 55% depending on the subgroup). Specifically, the prevalence is 10% (95% confidence interval [CI]: 7, 12) among 30- to 49-year-old men; 17% (95% CI: 15, 21) among 50- to 70-year-old men; 3% (95% CI: 2, 4) among 30- to 49-year-old women; and 9% (95% CI: 7, 11) among 50- to 70-year-old women.3
There is increasing awareness of sleep disorders by the general public and respiratory physicians, by necessity, are dealing more and more with sleep apnea and other sleep disorders. In recognition of the need for training pulmonary physicians in sleep disorders, in 1994 the American Thoracic Society (ATS) published recommendations for training in sleep medicine that has grown to include not only the ATS, but also the AASM, ACCP, AASM,4 and ABIM.5 There is also significant impact on future training in medicine and surgery based on the sleepiness of the learner. Restriction of duty hours has been reviewed in many countries including ACGME in the US and the RCPSC in Canada. The IOM Report Sleep Supervision and Safety reviewed the potential impact of sleep in training and recommendations made in 2008 were implemented by ACGME including:
The new ACGME standards require residency programs to:
1. Tailor supervision standards for different levels of training, particularly greater supervision for first-year residents.
2. Ensure competence in structured handover processes.
3. Incorporate clinical quality improvement and patient safety into resident learning.
4. Provide safe transportation and/or sleeping facilities for fatigued residents.
5. Adjust workload according to patient severity and resident training.
6. Improve oversight of compliance with duty hour limits.
Specific duty hour recommendations from the IOM include:
1. The maximum number of work hours remains at 80 hours per week, averaged over 4 weeks;
2. Moonlighting, now both internal and external, is counted against the 80-hour weekly limit; and
3. Duty periods are limited to 16 hours (although only for first-year residents by ACGME).
In Canada, the author (MS) was a member of the Medical Education Working Group and contributing author of Fatigue, Risk, Excellence: Towards a Pan-Canadian Consensus on Resident Duty Hours (June 2013). The recommendations from this report suggest each training program is unique and therefore deserves unique solutions for managing sleep schedules to optimize learning. The National Steering Committee published five recommendations for Canadian training programs:
1. Recognizing that there are many factors that contribute to resident fatigue, a comprehensive approach to minimize fatigue and fatigue-related risks should be developed and implemented in residency training in all jurisdictions in Canada.
2. Educational approaches should be redesigned to leverage innovations and new approaches, to ensure appropriate training and acquisition of competencies in an era of increasing resident duty hour regulations.
3. Accreditation standards must be adapted to support planned modifications of the content and duration of resident duty, through the enforcement of fatigue risk management activities.
4. An inventory of alternate models of scheduling and after-hours care provision should be created and disseminated to provide alternatives and benchmarks of scheduling and service delivery.
5. An independent, pan-Canadian consortium devoted to the evaluation of resident duty hours in Canada should be created.
These organizations are looking to guidance from physicians with sleep training to help mitigate sleepiness to optimize learning. There are many additional public health issues including patient safety that require physicians to understand sleepiness.6 It is clear, therefore, that pulmonary physicians need to better understand and be able to treat EDS, regardless of the etiology.
THE PHENOMENON OF SLEEPINESS
Sleepiness is both a subjective and an objective phenomenon, a constellation of sensations and a physiological state with stereotypical behaviors. As such, it is sometimes difficult to define, and its measurement (see Section on Quantifying Sleepiness) depends on the circumstances. Sleepiness may be expressed as feeling sleepy, fatigued, or tired; sleeping too much; or fighting to maintain alertness. Sleepiness can be reflected by any or all of the following: heaviness of the eyelids, mild burning or itching of the eyes, difficulty keeping the eyes open, heaviness in the arms or legs, reluctance to move, loss of initiative, loss of interest in surroundings, and difficulty with concentration or memory. These sensations are accompanied by behavioral changes such as rubbing the eyes, yawning and stretching, and nodding the head, and by generally reduced motor functions such as speech, facial expression, and body movement. Literature shows that sleep-deprived healthy individuals demonstrate attenuated facial expressions to stimuli.7
Sleepiness may also be considered a physiological state like hunger or thirst. Just as hunger and thirst are physiological states that occur with fasting and are satisfied by eating and drinking, sleepiness in individuals without a specific sleep disorder is produced by sleep restriction or deprivation and is reversed or satisfied by sleep. However, a difference between sleep and other physiological factors is that food may satisfy hunger but the sleep quality along with quantity is necessary to satiate sleep loss. Sleep quality is impaired in individuals with specific sleep disorders such as sleep apnea. The factors that produce and influence sleepiness are detailed below; they include such obvious factors as time since last asleep, previous amount of sleep, continuity of sleep, and normal 24-hour circadian influences. Environmental stimuli influence this state and can determine, up to a point, whether or not this sleepy tendency will be manifested. For example, heavy meals, warm rooms, boring lectures, or monotonous tasks are usually considered soporific activities or situations. In these situations, a person might feel sleepy and, might fall asleep. Yet the environmental factors themselves do not cause the sleepiness; they only allow it to be expressed. Equally, the same degree of physiological sleep tendency might go unnoticed when environmental stimulation occurs in the form of a life-threatening situation. In other words, the degree to which sleepiness is experienced or evident in behavior is determined by the underlying physiological sleep tendency (or the need for sleep) and environmental factors, which interact to manifest the sleep tendency or sleep propensity.8
While it is accepted that sleepiness is a physiological state, the physiological substrates of this state have not been identified. Neurotransmitters such as serotonin, acetylcholine, histamine, and the catecholamines have been implicated in the sleep/wake mechanism along with a variety of other sleep-inducing substances, including adenosine through its inhibition of wakefulness-promoting neurons. While much research is ongoing, the understanding of the neurochemicals responsible for sleep, sleepiness, and loss of alertness are still far from clear.9–13
QUANTIFYING SLEEPINESS
The sensation of sleepiness is often difficult to quantify, as are other subjective symptoms, such as pain or shortness of breath. All of these subjective sensations may mean different things to different people, and are modified by factors including motivation, external stimulation, and competing needs. What constitutes extreme sleepiness for one person may be only mild sleepiness for another and depends on the situation in which it occurs. An approach is outlined in Table 102-1. Sleepiness has different dimensions with both feelings of perceived sleepiness as well as self-estimates of sleepy behavior, which are different in passive versus active situations. The notion of a sleepiness trait, a composite of sleep need and sleepability, which is the ability to transition into sleep.14
Other individual difference factors have been proposed to explain individual dissimilarities in sleepiness.8 Sleep drive is an inherited trait.15
 SUBJECTIVE MEASURES OF SLEEPINESS
SUBJECTIVE MEASURES OF SLEEPINESS
Subjective reports may be used to quantify sleepiness, but statements such as “I feel sleepy” and “I feel very sleepy” often do not distinguish between feelings caused by a high physiological sleep tendency and those resulting from muscular fatigue, depressed mood, or a general lack of energy. Thus, several subjective sleepiness scales have been developed. The Stanford Sleepiness Scale (SSS), the first to receive widespread use, is a seven-point self-rating scale ranging from 1 (alert, wide awake) to 7 (almost in reverie, sleep onset soon).16 It is brief, simple to use and measures current degree of sleepiness. It has been shown to correlate with the performance of mental tasks and demonstrate changes in sleepiness with sleep loss. However, there are no normative data and results often depend on the duration of prior sleepiness. For example, unlike normal persons who are experimentally sleep deprived, patients with more chronic sleep deprivation (i.e., sleep apnea) cannot be accurately tested with the SSS. Some patients who have an obvious overwhelming physiological sleep tendency may claim to be only mildly sleepy, yet fall asleep before your eyes. This was first observed in the early 1970s, and was a stimulus for Stanford investigator group to develop more objective measures of sleepiness.16 Studies have shown that with continued increases in lapses in performance over several days of sleep restriction but the self-reported sleepiness does not continue to get worse.17
It is clear that over a period of months or years, many sleep apnea patients lose their frame of reference with regard to normal alertness and cannot distinguish major changes in sleepiness. Thus, the subjective report of sleepiness (using the SSS) by people who are chronically and severely sleep deprived is not reliable.16
The Karolinska Sleepiness Scale (KSS) is a nine-point scale ranging from 1 (very alert) to 9 (very sleepy, fighting sleep, making an effort to keep awake), with verbal descriptions of every second point.18 Like the SSS,16 the KSS requires the subject to integrate and translate a number of sensations to a continuum that is fairly abstract despite the verbal description. Ratings obtained with these scales may be affected by the situation in which the scale is presented (at rest or during performing a task) and how the subject relates his or her perception to that particular time or place. These scales reflect current sleepiness. Nonetheless, both the SSS and KSS show high correlations with performance. The KSS was also found to be strongly related to EEG and electrooculographic signs of sleepiness.18
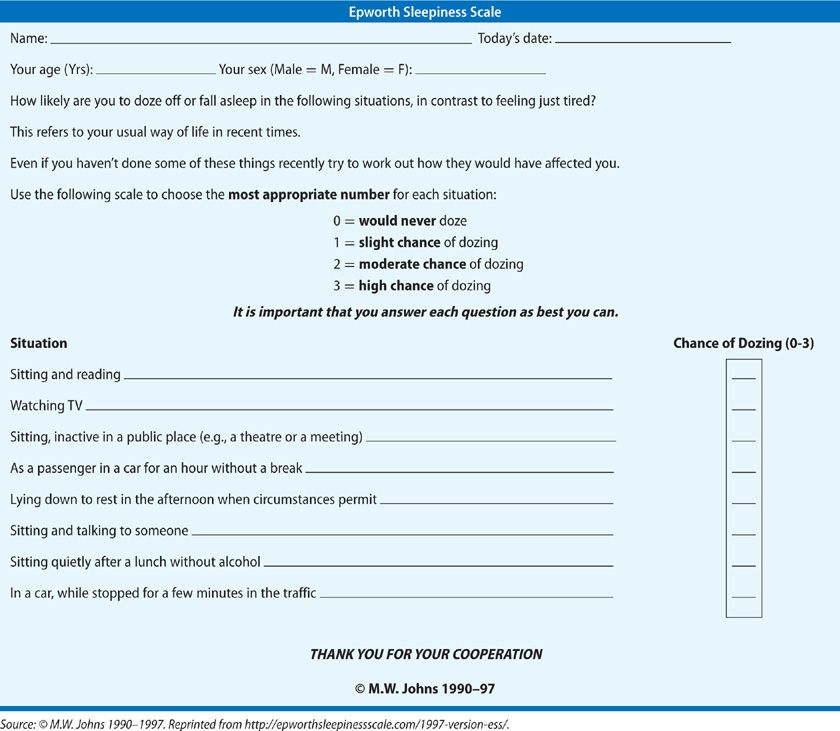
The Epworth Sleepiness Scale (ESS; Table 102-2) was designed to measure sleep propensity in a single, standardized way and is based on questions relating to eight situations, some known to be very soporific.19 The ESS, in contrary to the KSS and SSS, assesses symptoms of chronic sleep deprivation. The questions are self-administered, and subjects are asked to rate on a 0 to 3 scale how likely they are to doze off in the situation based on their usual habits over recent weeks to months. The ESS tries to overcome the fact that people have different daily routines, some facilitating and others preventing daytime sleep. An ESS of 10 or below is considered normal. Scores above this are considered indicative of excessive sleepiness. ESS scores have shown significant correlations with mean sleep latency in the multiple sleep latency test (MSLT, see further below) and have distinguished groups of patients with disorders of excessive sleepiness such as narcolepsy, OSA, and idiopathic hypersomnolence. The ESS score has been validated against the MSLT in sleep disorder patient population, specifically narcolepsy patients, to show a 95% sensitivity and 73% specificity in comparison to the MSLT.20 The ESS has high test–retest reliability and a high level of internal reliability in normals and patients with sleep apnea.21 Further work examining the utility of measuring sleepiness in different situations using the ESS suggests that individual measurements of sleep propensity (i.e., sleepiness) entail three components of variation: a general characteristic of the subject (the average sleep propensity), a general characteristic of the situation in which the sleepiness or sleep propensity is measured (its soporific nature), and a third component that is specific for both subjects and situation.
 OBJECTIVE MEASURES OF SLEEPINESS
OBJECTIVE MEASURES OF SLEEPINESS
MSLT20 has been developed and standardized as an objective, reliable, and reproducible measure of physiological sleep tendency.20 Performed at intervals throughout the day the MSLT measures the time to sleep onset, as determined by the EEG. This test is based on the assumption that, given the proper surroundings, physiological sleep tendency will be expressed; it has an intuitive appeal in that if one patient is more sleepy than the other, the sleepier patient should fall asleep more quickly. Patients are instrumented to record the EEG, electrooculogram (EOG), and electromyogram (EMG); they are put in a quiet, darkened, temperature-controlled room, and are asked to lie quietly, close their eyes, and try to fall asleep. Naps are scheduled at 2-hour intervals, with 20 minutes allowed for sleep to occur. If an individual initiates sleep, a mean sleep onset latency (SOL) can be measured and can support excessive daytime somnolence or a diagnosis of narcolepsy, as explained below. Thus, the average SOL of the naps represents the result of the MSLT. Both clinical and research protocols exist for conducting the MSLT.20
Since sleepiness follows a circadian rhythm (see Section on Circadian Rhythms), one nap is insufficient to document and quantify daytime sleepiness. Accordingly, a minimum of four and a maximum of six naps are recommended. The MSLT is a reliable, reproducible test that has been validated in a number of sleep deprivation experiments in normal subjects and a variety of clinical conditions with patients who have disorders such as narcolepsy and sleep apnea and may be useful for documenting treatment response to the sleep disorder, such as medications in narcolepsy or CPAP in sleep apnea.20 An important advantage of the MSLT is that patient motivation cannot counteract the effects of previous sleep loss on sleep latency. That is, while most people can be motivated to compensate for reduced performance after sleep deprivation, motivation cannot overcome an increased pressure for sleep, particularly when the patient is in bed in a darkened room.
The Maintenance of Wakefulness Test (MWT) is an alternative to the MSLT. This is a variation on a theme in which subjects sit in a chair in a darkened room and are requested to remain awake for 20 (or 40) minutes. This test was developed on the assumption that the ability to fall asleep and the ability to stay awake are two separate phenomena. The MWT has undergone further tests of validity, but has been criticized for lack of a standardized protocol with 20-, 30-, and 40-minute tests being reported. Recent practice parameters suggest using a 20-minute four-trial protocol opportunities for the patient to initiate a nap.20 The naps are separated by 2 hours.
This is a relevant question when assessing whether or not a patient can stay awake in a situation of personal or public safety. The physiological ability to fall asleep is measured by any naps initiated during the 20-minute period. Any sleep onset REM (SOREM) is closely observed as it can signify a diagnosis of narcolepsy.
While both MSLT and MWT require observer recognition of EEG changes, quantitative computerized analysis of EEG has been proposed as an alternate and perhaps more sensitive objective measure of sleepiness. Increased EEG delta activity with sleep deprivation and decreased alpha activity just before sleep onset are two possible metrics. However, these have yet to be translated into clinically useful tests. The Alpha Attenuation Test (AAT) has been validated in sleep-restricted normals and in patients with narcolepsy and correlates strongly with the MSLT.20
Oxford SLEep Resistance Test (OSLER test) was designed as a low-cost alternative designed to reproduce many of the features of the MWT, but without the labor-intensive, continuous technician monitoring of EEG.22
In the OSLER tests, subjects respond to a visual stimulus. A light-emitting diode mounted on the wall, which flashes for 1 second every 3 seconds in fixed intervals. If there is no response after seven consecutive stimuli, the subject is deemed to be asleep and the test is ended. The original study compared OSLER sleep latency with MWT latency in 10 OSA patients and 10 control subjects, done on separate days, and showed that OSLER could be validated against the MWT in this patient population. Two other studies involving small numbers of sleep disorders center patients and/or normal subjects before and after sleep deprivation have demonstrated excellent agreement between the two measures and suggest that the OSLER could be an alternative to measuring sleepiness.23 In a study of heart failure patients receiving adaptive ventilation for treatment of Cheyne–Stokes respiration, improvement in OSLER scores followed improvement in nighttime sleep.24 Despite these promising results, there are no large-scale studies using the OSLER, and the main limitation of this test is its dependence on patient cooperation.25,26
 PERFORMANCE AND VIGILANCE TESTS
PERFORMANCE AND VIGILANCE TESTS
Measurements of performance after sleep loss reflect daytime sleepiness, since most people report decreased performance after a sleepless night. Previously it was felt that only performance tests that were prolonged and monotonous were sensitive to sleep loss. However, the work of Dinges et al. (using his Psychomotor Vigilance Task [PVT]) demonstrates that if the signal rate is high and the response measure sufficiently sensitive, repetitive tasks of only 10-minutes duration will expose the limits of performance in sleepy persons.27,28 Performance decrements resulting from sleep deprivation (or sleep disorders such as sleep apnea) can be observed in such a task if results are analyzed over time. This time-on-task or vigilance decrement may be observed as evidence of fatigue even in well-motivated subjects with adequate prior sleep, and it manifests itself as a shallow decline in performance as time-on-task increases. When the subject is sleep deprived, it is impossible to sustain attention long enough to maintain peak performance throughout the entire task and lapses in performance occur. Sleep loss increases the rate of decline in performance. The number of interrupted episodes also contribute to performance decline. The Psychomotor Vigilance Test (PVT) has become the most widely used measure of neurobehavioral performance. It has been validated with the SSS and MSLT. Recent work using this test has demonstrated consistent individual differences in neurobehavioral deficits from sleep loss when repeated in the same subject, which indicate differential trait vulnerability to sleepiness.29
While the MSLT and MWT can provide helpful information, they require repeated testing every 2 hours and a whole day of tests. Data published recently suggest that a single administration of objective tests, in particular the PVT, can help assess sleepiness in controlled conditions that may be applied clinically.30
Other tests of sustained attention and performance have been developed, many to assess simulated driving performance. Using a divided attention task, where individuals are required to pay attention to more than one aspect of the task, such as a driving task, sleep apnea patients have been shown to perform poorly, and in some cases, equal to or worse than normals impaired by alcohol.31 Nonetheless sleepiness as measured by MSLT accounts for less than 25% of the variance in tracking performance. Thus, while the effects of sleepiness on performance may occur in a dose-dependent fashion in normals, performance decrements in patients who are sleepy because of an underlying sleep disorder may be accounted for by factors other than sleepiness.
Mitigating subjective “sleepiness” in patients with mild to moderate OSA, untreated with CPAP, has been shown with modafinil. The effect size was clinically important as was the improvement on the driving simulator performance and reaction time.32
FACTORS AFFECTING SLEEPINESS
Sleepiness is determined by the quantity of sleep and the quality and type of sleep, interacting with circadian rhythms or drugs that patients may be taking.
 SLEEP QUANTITY
SLEEP QUANTITY
The amount of nocturnal sleep has a strong relationship to the degree of daytime sleepiness. Partial or total sleep deprivation is followed by increased daytime sleepiness in normal persons. Furthermore, sleep restriction will become cumulative over time and lead to increasing daytime sleepiness.28
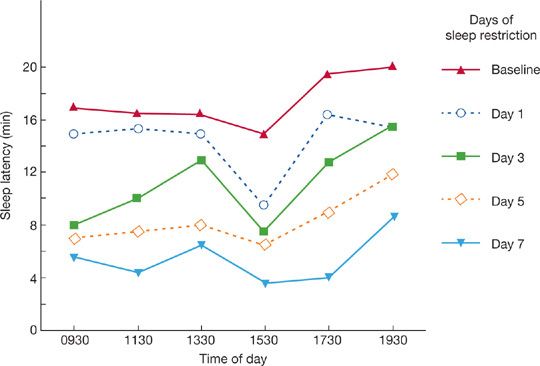
The effect of sleep restriction on SOL is shown in Figure 102-1. When the sleep of young adults was reduced by 2 hours a night on consecutive nights, sleepiness (as measured by the MSLT) progressively increased over 7 days. Even as little as 1 hour per night of sleep loss will accumulate over time and lead to daytime sleepiness—a fact generally not appreciated. Each person has a certain biological sleep need, and the specific amount varies from one subject to the next. Regardless of cultural or environmental factors, most adults sleep 7 to 8 hours per day, but the old adage that we must sleep 8 hours each night is not true for everyone. Some people require more than 8 hours, and others less. There may be many contributors to the amount of sleep an individual requires including a genetic contribution. Recent data support a genetic variant (hDEC2-P385R) contributing to individuals who only need a short sleep time.33 Individual differences in required sleep time is also evident in conjoined twins who show an independence of sleep needs. In the absence of pathology, normal human sleep length varies between 6 and 9 hours, although some people require less. It would be ideal to require a minimum amount of sleep to allow maximum productivity in work and adequate time for social pursuits. Indeed, some investigators believe that Western society predisposes to sleep deprivation.34 With economic and social constraints – the latter leading to voluntary sleep restriction – the sleep period is the time most encroached on, potentially leading to daytime sleepiness.35 There has been progressive reduction in sleep duration over many age ranges that is more commonly found in the United States. There are multiple etiologies for this including work intruding into sleep period. Thus, there is a national agenda for 2020 (Healthy People 2020) in the United States to understand the implications and associations of this more comprehensively.36 Overall, it appears short sleep duration is increasing in prevalence, which may be associated with adverse health outcomes, such as weight gain and associated morbidities of obesity.37 In addition, the prevalence of short sleep (<6 hours) has increased in individuals who are employed full time.38
Figure 102-1 Average daily sleep latency test scores for young adults when nighttime sleep was reduced by 2 hours a night for 7 consecutive nights. (Adapted with permission from Dement WC, Carskadon MA. An essay on sleepiness, in Boldy-Moulinier M (ed). Actualitiés en Médécine Expérimentale, en Hommage au Prof D Passouant. Montpellier, Euromed; 1981.)
Voluntary sleep restriction or insufficient sleep causes daytime sleepiness. Among all prominent features differentiating this group of patients with insufficient sleep from those with narcolepsy is the report, obtained from the sleep history, of a disparity between the amount of sleep on weekdays and that on weekends. People with insufficient sleep typically have a much longer sleep period on weekends (by 2 hours or more).
Most patients consider their weekly sleep loss trivial and assume that it is recovered on weekends. However, while recovery from a single experimental sleep restriction occurs in a couple of nights, it is not likely that repeated episodes of sleep deprivation can be compensated for in just one night. A study of a large group of normal subjects without complaints of daytime sleepiness has shown that young subjects (particularly college students) had shorter sleep latencies than did older subjects. Within the group of 120 young subjects, 12 healthy, nonsmoking men aged 21 to 35 years, had a mean sleep latency of less than 6 minutes on MSLT testing, whereas another 12 had an MSLT of greater than 16 minutes.20 These subjects had baseline testing and then extended their sleep period time from 8 to 10 hours over 6 days. Repeat testing on days 1, 3, and 6 showed stepwise increases in MSLT and performance testing for both subgroups. These data support the notion that chronic voluntary sleep restriction in real life produces objective sleepiness that may or may not be perceived by the subject.
 SLEEP QUALITY
SLEEP QUALITY
Sleep quality is perceived to be abnormal when sleep is decreased or discontinuous. Disrupting sleep continuity – that is, causing arousal from sleep, either experimentally or by sleep disorders – affects the quality of sleep and results in increased physiological sleep tendency. An arousal can be defined as a brief (3–15 seconds) speeding up of the EEG, or as a burst of alpha activity occasionally accompanied by transient increases in skeletal muscle tone.11,12 These typically do not result in awakening as defined by standard sleep staging criteria or behavioral indicators. Sleep studies can identify various causes of arousal, such as recurrent obstructive apnea, periodic leg movements, or chronic pain, in some but not all cases. A common exception is the patient with chronic obstructive pulmonary disease (COPD) who has frequent arousals from sleep in the absence of obstructive apnea or leg movements.39 Patients with COPD often experience oxygen desaturation during sleep, and this is a potential stimulus for arousal. Nonetheless, compared with age-matched controls, COPD patients have discontinuous sleep and poor sleep efficiency (defined as percentage of time actually asleep in bed).
Auditory stimuli presented externally to normal subjects during sleep can produce arousal; repetitive presentation of such stimuli can produce daytime sleepiness. Several studies have shown decreased performance and increased sleepiness the day after repetitive arousal, with the degree of daytime sleepiness related to the frequency of nocturnal sleep disruption. Not surprisingly, the shortest sleep latency occurred after the most fragmented nocturnal sleep. This increased sleepiness will result even if the stimulus is only sufficient to produce EEG signs of arousal, without full wakefulness. The importance of sleep disturbance from environmental noise has been reported by Basner et al.40 There are both auditory effects such as EEG arousals, impaired daytime alertness from lack of sleep quality and quantity as well as quality of life and nonauditory effects, such as increased catecholamine release and association with cardiovascular disease. Environmental noise exposure, such as from airports and other urban environmental areas, can cause lasting damage resulting in hearing loss. Noise-induced hearing loss and tinnitus may not always be recognized in our society but the effects on the affected individuals’ quality of life can be tremendous.40
 CIRCADIAN RHYTHMS
CIRCADIAN RHYTHMS
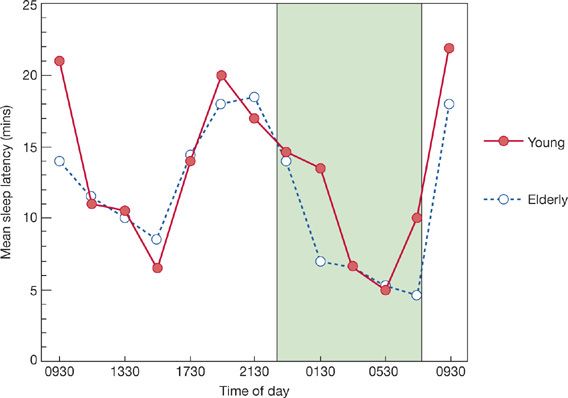
If sleep latency is measured every 2 hours over a complete 24-hour day, a biphasic pattern of sleep tendency becomes obvious (Fig. 102-2). This demonstrates that there are two peaks and troughs of sleepiness over a 24-hour period. Not surprisingly, the times of increased sleepiness are during the nocturnal hours and during the daytime hours (in the midafternoon between 2 and 4 PM). This circadian rhythm of sleepiness is present in all age groups, although the time of the peak rhythm may vary. The circadian rhythm of sleepiness is similar to other circadian rhythms in that it possesses an endogenous periodicity that can be affected by environmental influences that fine tune or entrain the rhythm. Even in the absence of these environmental cues (i.e., awakening time, alarm clock, degree of light or darkness, food and stimulants, and social contact), rhythms show a persistent periodicity. The circadian rhythm of temperature is extremely stable. Temperatures fall in the late afternoon, are lowest during the middle of the sleep period, and rise before morning awakening. The temperature rhythm synchronizes most closely with sleepiness. Although the amplitude of body temperature and sleep latency rhythms differs considerably, no other biological rhythms correlate so well in time. Circadian rhythm is discussed more extensively in Chapter 12.
Figure 102-2 Sleep latency (mean) as a function of time of day for young subjects (filled circles) and elderly subjects (open circles). Stippled area denotes nighttime sleep period. (Adapted with permission from Richardson GS, Carskadon MA, Orav EJ, et al. Circadian variation of sleep tendency in elderly and young adult subjects. Sleep. 1982;5(Suppl 2):S82–S94.)
Two other examples of the influence of circadian rhythms on sleepiness are obvious. The first is that associated with shift work, and the second is due to transcontinental travel (jet lag). Workers with a normal nocturnal sleep period and a previously stable circadian sleepiness rhythm suddenly will have a trough of sleepiness during the middle of their night work period. They will attempt to stay awake, while the circadian influences will promote sleep.27,41 Jet lag is similar, in that the body’s circadian rhythm is out of synchrony with the destination clock time. As a result, performance may suffer.
 MEDICATIONS
MEDICATIONS
Drug effects on sleep can be significant and can either promote sleep and sleepiness or increase wakefulness and alertness. When assessing subjective or objective sleepiness, it is important to know whether the patient is taking a medication that may affect the degree of sleepiness, that is, sleepiness is a side effect of this medication.
Not surprisingly, sedative drugs increase sleepiness. Benzodiazepine hypnotics have been used to help people get to sleep at night however nonbenzodiazepine hypnotics are also used. Many objective studies confirm the ability of hypnotics to shorten sleep latency at bedtime through GABAA receptor stimulation. When given during the day, they will promote sleep. However, the daytime carryover effect of nocturnal sedation is not always recognized. This effect occurs most commonly with long-acting benzodiazepines, but it may occur with other medications as well. Recently, warnings have been issued from the FDA about potential sleepiness the morning after taking these medications, especially in women, and have recommended reduction in zolpidem dosages to half to increase patient safety. Second-generation antiepileptics (i.e., gabapentin, pregabalin, vigabatrin) also act as GABA agonists without interacting with the GABAA or GABAB receptors. Alcohol consistently shortens sleep onset and produces sedation, whether given at night or during the day. It, however, also alters sleep architecture by suppressing REM sleep and contributing to sleep fragmentation through the latter portions of the night.42
Drugs that produce sleepiness include antihistamines, which are used in allergy and pulmonary practice. Many of the early H1 antihistamines, such as diphenhydramine and chlorpheniramine, have been shown to reduce the MSLT. Some newer antihistamines, such as terfenadine and astemizole, do not produce objective sleepiness. The more lipid-soluble drugs (i.e., diphenhydramine and chlorpheniramine) penetrate the central nervous system more easily and therefore are more likely than less lipid-soluble drugs to produce sedation. Other medications with high lipid solubility have been reported to produce daytime sedation; the most common of these are the beta-blocker drugs. There are no controlled, objective studies of sleep latency with this type of drug, and sleepiness from these medications is based on reports of side effects or subjective questionnaires, such as Pittsburg Sleep Quality Index (PSQI).43
The effect of a particular drug in producing sleepiness also depends on the background level of sleepiness or alertness. When ethanol or caffeine is given to normal-sleeping young men in the morning, one might expect ethanol to produce daytime sleepiness and caffeine to increase sleep latency during the day. Subjects are consistently sleepier after ethanol than after caffeine ingestion, but fully rested subjects (those having spent 11 hours in bed) do not show sleepiness after taking ethanol. In other words, the sedative effects of drugs such as alcohol can be enhanced by increased background sleepiness. Thus, a driver who is sleepy to start with may be as vulnerable after just one or two drinks as a previously alert driver who has become legally intoxicated.42
Drugs that increase wakefulness or alertness include stimulants such as amphetamine, methylphenidate, modafinil, and armodafinil. These are most often used in the treatment of narcolepsy but may be used to stay awake for long periods of time. Caffeine, probably the most widely used stimulant, can reduce daytime sleepiness and transiently increase alertness. Excessive caffeine intake also paradoxically may cause a degree of daytime sleepiness. This occurs when caffeine levels persist into nocturnal hours and promote difficulties with sleep onset and increased awakenings during sleep.
In patients with narcolepsy, another agent, sodium oxybate (Xyrem) is used to treat EDS and cataplexy through action of GABAB and gamma-hydroxybutyrate (GHB) receptors.44 While its mechanism of action is not completely clear, it has been shown to dramatically increase the amount and duration of slow-wave sleep in patients. While it is only given at night, due to its rapid onset of sedation, interestingly, the medication promotes alertness through the day shown in randomized trials.45
 PREVALENCE OF EXCESSIVE DAYTIME SLEEPINESS
PREVALENCE OF EXCESSIVE DAYTIME SLEEPINESS
Prevalence rates for sleepiness depend greatly on the type of questions addressing sleepiness. Are you sleeping too much versus are you falling asleep during the daytime versus does your sense of sleepiness impair your daytime activities all result in widely different prevalence rates. Also men tend to report sleepy behavior whereas women report feelings of EDS, again contributing to variable prevalence. Prevalence of sleepiness also varies with the population examined.
There are many socioeconomic and demographic factors that may contribute to sleep disorders or poor sleep quality or quantity in the population. Specific associations with long SOL include being female, having black/African American heritage, lower education, lack of private health insurance, as well as food insecurity. The demographics were most associated, not only with prolonged SOL but with nonrestorative sleep as well as sleep maintenance difficulties, including early morning awakenings. EDS occurred more frequently in female gender and in divorced individuals.46
The Wisconsin Sleep Cohort study47 was the first to formally determine the prevalence of sleepiness as a function of sleep apnea. This landmark study demonstrated that at least 2% of middle-aged women and 4% of middle-aged men had OSA and symptoms of EDS. More recent literature suggests 14% to 55% of the population has Obstructive Sleep Apnea Hypopnea Syndrome (OSAHS).3 While there may have been other causes for the daytime sleepiness besides OSA, it is clear that sleep apnea is responsible for a great deal of the daytime sleepiness in North America.
EVALUATING THE SLEEPY PATIENT
Keeping in mind the factors that determine daytime sleepiness, the sleep history can be individualized and can be very helpful in narrowing the differential diagnosis (Table 102-3). One should always question the patient about his or her nocturnal sleep, looking specifically at sleep onset time, sleep period time, number of awakenings, and time of rising in the morning. One should also ask about when the change in sleep occurred as well as questions about sleep hygiene (i.e., when awake, reading book in dim light vs. playing games on an electronic device with bright light). Sleep onset phenomena such as sleep paralysis and hypnagogic hallucinations often suggest a diagnosis of narcolepsy although these sometimes occur in apneics who are severely sleep deprived.48 A history of loud snoring or stopping breathing during sleep is suggestive of sleep apnea hypopnea syndrome, particularly if the snoring is “cyclical” rather than continuous, with periods of loud snoring or snorting alternating with quiet intervals. Since insufficient sleep may be the cause of sleepiness, it is important to ask if there is any difference in the amount of sleep required during the week compared with that on weekends. Equally important is whether the patient has any changes in subjective sleepiness on weekends or holidays compared with weekdays.