Introduction
Diet, as regularly consumed, and the nutrients supplied by it are major determinants which initiate and influence the course of atherothrombotic vascular disease.1 It is estimated that up to 30% of deaths from coronary heart disease (CHD), a major form of cardiovascular disease (CVD), are due to unhealthy diets.2 Identification of increased or decreased risk associated with dietary patterns or specific nutrients, in a methodologically rigorous manner, should lay the scientific foundation for general dietary recommendations to populations as well as specific nutritional interventions in individuals at high risk of CVD.
Methodologic issues in the study of causal associations
Issues related to study design
Studies investigating the influence of diet on CVD or cardiovascular risk factors have employed a wide variety of study designs: ecologic studies within and across populations, cross-sectional surveys, case–control studies (de novo or nested), cohort studies, community-based demonstration projects, randomized clinical trials and before/after type of metabolic studies. These differ widely in terms of their ability to (a) identify, avoid and adjust for confounding, (b) establish a temporal relationship of cause preceding the effect, (c) minimize bias, (d) provide a wide range of exposure, (e) ascertain composite endpoints, including fatal outcomes, (f) evaluate population-attributable risk, and (g) yield generalizable results.
Clinical trials, if well designed, provide the best framework for studying associations, as the results are relatively free from bias and confounding. However, they often evaluate interventions which are relatively short term and introduced late in the natural history of disease and may not reflect the effects of long-term differences in dietary exposures. Genetics now offers an alternative approach through “Mendelian randomization”. This approach takes into account that genotypic differences in the metabolism of food ingredients may cause lifelong differences in exposure to food components and their metabolites or to purported risk factors, and may thus establish causality without the need for prolonged follow-up.3,4
A related issue is the use of experimental animals. Although these are often referred to as “animal models”, their validity in predicting outcomes in humans is unclear. Lipid metabolism especially is species specific, as exemplified by the lack of efficacy of cholesterol-lowering statin drugs in many animal species, including monkeys.5 Experiments in animals are therefore best reserved for elucidating mechanisms, and cannot be used to argue that a particular food will have a particular effect on cardiovascular disease in humans.
Issues involving outcome variables
These principally relate to a choice between disease end-points and intermediate variables and the type of variables which are selected for study. Ideally, disease-related end-points are preferable since they clearly demonstrate the benefits or risks of dietary exposures. With an exposure such as diet, effects may extend beyond cardiovascular outcomes. The need to evaluate impact of diet on total mortality and major co-morbidities, therefore, becomes important. Dietary exposures which influence thrombotic pathways may have opposite effects on the risk of hemorrhagic stroke and thrombotic stroke. The need to differentiate the types of stroke in outcome evaluation is, therefore, clear and has important implications for populations which differ in their stroke profiles.
Intermediate variables have been frequently utilized in studies evaluating the association of dietary constituents or dietary patterns to CVD. Most often, these are risk factors like blood pressure or plasma lipids. However, similar changes in total plasma cholesterol may be associated with variable effects on levels of LDL cholesterol and HDL cholesterol and on the ratio of total to HDL cholesterol. The impact on risk of atherosclerotic CVD may thus vary. The 25-year follow-up experience of the Seven Countries Study revealed that while the increase in relative risk of CHD for comparable levels of plasma cholesterol elevation was similar across diverse populations, the absolute risk of CHD varied widely at the same level of plasma cholesterol, possibly due to other dietary and non-dietary influences.6 Dietary changes may also influence LDL particle size differentially, and also the level of plasma triglycerides, with variable net effects on the atherogenicity of the plasma lipid pool. Such limitations were clearly illustrated in a study by Rudel et al7 in which monkeys fed monounsaturated fat had similar lowering of LDL cholesterol as monkeys fed polyunsaturated fat but developed atherosclerosis equivalent to those fed saturated fat. In monkeys fed monounsaturated fatty acids, there was an enrichment of cholesteryl oleate in plasma cholesteryl esters, which correlated with coronary artery cholesteryl ester concentration.8 Plasma lipids, as intermediate variables, could not also explain the degree of cardiovascular protection conferred by the Mediterranean diet in the Lyon Diet Heart Study.9 While studies of intermediate variables (e.g. cholesterol levels) are useful in identifying mechanistic pathways of diet, there is a need for methodologically strong studies which relate dietary patterns or dietary interventions to hard endpoints such as total mortality and combined fatal and non-fatal cardiovascular events.
Issues involving exposure variables
These involve the type of exposure selected for study, the methods of measurement employed as well as the duration and dose of exposure. First, the types of dietary exposure assessed for associations with CVD have varied from specific nutrients (such as saturated fat) to dietary items (such as fish) to food groups (such as fruit and vegetables) to dietary patterns (such as the Mediterranean diet or Adventist diet) and composite dietary interventions (e.g. the DASH diet). A reductionist approach has inherent limitations in the area of diet, because multiple interactions among many nutrients are likely to determine the physiologic effects and pathologic outcomes much more than the individual effects of an isolated nutrient. Multicomponent dietary exposures, however, render identification of mechanistic causal pathways difficult to elucidate. While this frustrates efforts to develop and market specific food supplements or nutriceuticals, interests of public health are likely to be better served by a causal enquiry exploring the connections of food patterns and food components to cardiovascular health.
Second, the strengths and limitations of various methods of collecting accurate food consumption data are well recognized.10 Questionnaire methods of ascertaining information related to habitual food intake pose problems of validity and reproducibility even within well-defined populations but these problems are likely to be magnified when such instruments are applied across different cultures. Even if the nutrient composition of self-reported diets is accurately estimated, different cooking methods may alter the final bio-availability of those nutrients as actually consumed. The need for valid and reproducible biomarkers is, therefore, important when studies of specific nutrients are proposed. For example, adipose tissue fatty acid composition is a suitable biomarker for habitual type of dietary fat intake.11 There may, however, be technical and financial constraints which limit the use of such bio-markers in large epidemiologic studies.
Third, a causal enquiry needs to recognize the lag time effect, wherein a long period of exposure to dietary variables is required before effect is evident on outcome variables Short-term studies may be incapable of identifying true effects even when they exist. This is clearly illustrated by trials evaluating the effect of sodium restriction on blood pressure, where benefit was demonstrated only in trials in which the duration of exposure was at least 5 weeks.12 The dose of exposure is another critical variable, where many of the nutrients are physiologic requirements at a certain level but may pose risk of cardiovascular dysfunction and disease at other levels. The relationships may vary from linear to J-shaped or threshold, for different variables.
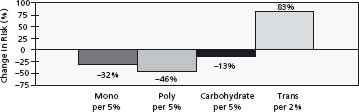
Figure 23.1 Change in CHD risk associated with replacement of saturates by other fats (Nurses’ Health Study). (Reproduced with permission from Hu et al21.)
Issues related to diet as an independent variable
Unhealthy dietary behaviors often occur in association with other unhealthy behaviors such as physical inactivity and smoking. Furthermore, unhealthy dietary practices such as high consumption of saturated fats, salt and refined carbohydrates as well as low consumption of fruit and vegetables tend to cluster together. In contrast, persons who habitually adopt one healthy dietary practice are more likely to adopt other healthy dietary habits as well as practice regular physical activity and abstinence from smoking. Dietary behaviors may also reflect patterns influenced by social class and may be influenced by stress levels. Dissociating the specific effects of individual dietary components from other dietary components, physical activity levels and other behaviors becomes difficult outside the setting of a randomized controlled clinical trial. In observational studies, the question arises whether some dietary practices are merely a surrogate for other dietary practices or lifestyles such as physical activity, or for a composite of multiple health behaviors.
The effects of diet on multiple cardiovascular risk factors, ranging from body weight to blood lipids and blood pressure to thrombotic mechanisms, also pose the question of when and how far to adjust for these variables in evaluating the relationship of diet to CVD. Since many of these are intermediate variables linking diet to CVD, adjustment to exclude their effect would underestimate the effect of diet. However, such variables are also influenced by factors other than diet.
Nutrients and cardiovascular disease
Willett defined a good diet by using a score based on low trans fat, high polyunsaturated fat, low glycemic load, high cereal fiber, fish twice a week or more, and high folic acid.13
Dietary fats
The relationship between dietary fats and CVD, especially CHD, has been extensively investigated, with strong and consistent associations emerging from a wide body of evidence accrued from animal experiments, as well as observational studies, clinical trials and metabolic studies conducted in diverse human populations. The relationship of dietary fat to CVD was initially considered to be mediated mainly through the atherogenic effects of plasma lipids (total cholesterol, lipoprotein fractions and triglycersides). The effects of dietary fats on thrombosis and endothelial function as well as the relationship of plasma and tissue lipids to the pathways of inflammation have been more recently understood.11,14 Similarly the effects of dietary fats on blood pressure have also become more evident through observational and experimental research.
Cholesterol in the blood and tissues is derived from two sources: diet and endogenous synthesis. Dairy fat and meat are major sources. Egg yolk is particularly rich in cholesterol but, unlike dairy and meat, does not provide saturated fatty acids. Dietary cholesterol raises plasma cholesterol levels.15 Although both HDL and LDL increase, the effect on the total/HDL ratio is still unfavorable, but small.16 Observational evidence on an association of dietary cholesterol intake with cardiovascular disease is contradictory.17,18 The upper limit for dietary cholesterol intake has been prescribed, in most guidelines, to be 300 mg/day. However, since there is no requirement for dietary cholesterol, it is advisable to keep the intake as low as possible.14 If intake of dairy fat and meat is controlled, there would be no need for a severe restriction of additional egg yolk intake.
Fatty acids are grouped into three classes – saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA). SFA and MUFA can be synthesized in the body and hence are not dietary essentials. PUFA are essential fatty acids, since they cannot be synthesized in the body.
Saturated fatty acids (SFAs) as a group raise total and LDL cholesterol, but individual SFAs have different effects.11,19 Myristic and lauric acids have greater effect than palmitic acid, but the latter is more abundant in food supply. The plasma cholesterol-raising effects of these three SFAs is higher when combined with high-cholesterol diets. Stearic acid has not been shown to elevate blood cholesterol and is rapidly converted to oleic acid (OA) in vivo. Metabolic (feeding) studies demonstrate a marked elevation of both HDL and LDL cholesterol induced by SFA diets.20,21 Replacement of saturated fatty acids by polyunsaturated fat reduces the total to HDL cholesterol ratio but replacement by carbohydrates does not. Also, tropical fats rich in lauric acid (C-12) raise total cholesterol strongly, but because of their specific effect on HDL, the ratio of total to HDL cholesterol falls. Thus effects on blood lipids can be variable, depending on which blood lipids are studied, and we need data on actual outcomes to determine the true effects of fats on coronary heart disease.22
The relationship of dietary saturated fat to plasma cholesterol levels and to CHD was graphically demonstrated by the Seven Countries Study involving 16 cohorts, in which saturated fat intake explained 73% of the total variance in CHD across these cohorts.23 In the Nurses’ Health Study,21 the effect of saturated fatty acids was much more modest, especially if saturates were replaced by carbohydrates. The most effective replacement for saturated fatty acids in terms of CHD outcome is by polyunsaturated fatty acids, i.e. linoleic acid. This agrees with the outcome of large randomized clinical trials, in which replacement of saturated and trans fats by polyunsaturated vegetable oils effectively lowered coronary heart disease risk.24
Trans fatty acids (t-FAs) are geometric isomers of unsaturated fatty acids that assume a saturated fatty acid-like configuration. Partial hydrogenation, the process used to create t-FAs, also removes essential fatty acids such as lin-oleic acid and alpha-linolenic acid. Metabolic studies have demonstrated that t-FAs render the plasma lipid profile even more atherogenic than SFAs, by not only elevating LDL cholesterol to similar levels but also decreasing HDL cholesterol.25 As a result, the ratio of LDL cholesterol to HDL cholesterol is significantly higher with a t-FA diet (2.58) than with a SFA diet (2.34) or an oleic acid diet (2.02). Evidence that intake of t-FAs increases the risk of CHD initially became available from large population-based cohort studies in the US26,27 and was corroborated in an elderly Dutch population.28 Levels of t-FAs in a biochemical analysis of replicated baseline food composites correlated with the risk of coronary death in the cohorts of the Seven Countries Study. Most trans fatty acids are contributed by industrially hardened oils, but the dairy and meat fats of ruminants are also a source. Eliminating t-FAs from the diet would be an important public health strategy to prevent cardiovascular disease. Since these agents are commercially introduced into the diet, policy measures related to the food industry would be required along with public education. Trans fatty acids have been eliminated from retail fats and spreads in a large part of the world, but deep-fat fried fast foods and baked goods are a major and increasing source.29
The only nutritionally important monounsaturated fatty acid (MUFA) is oleic acid, which is abundant in olive and canola oils and also in nuts. The epidemiologic evidence related to MUFAs and CHD is derived from studies on the Mediterranean diet, as well as from the Nurses’ Health Study and other similar studies, which investigated the association and controlled for confounding factors.30 MUFAs have been shown to lower blood glucose and triglycerides in type 2 diabetics and may decrease susceptibility of LDL to oxidative modification.
Polyunsaturated fatty acids (PUFAs) are derived from dietary linoleic acid (LA) (n-6 PUFAs) and dietary alpha linolenic acid (ALNA) (n-3 PUFAs). The important n-6 PUFAs are arachidonic acid (AA) and dihomogammalinolenic acid (DHGLA), while the important n-3 PUFAs are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Eicasanoids derived from AA have opposing metabolic properties to those derived from DHA. A balanced intake of n-6 and n-3 PUFAs is, therefore, essential for health.
The biologic effects of n-3 PUFAs are wide ranging, involving lipids and lipoproteins, blood pressure, cardiac function, arterial compliance, endothelial function, vascular reactivity and cardiac electrophysiology as well as potent antiplatelet and anti-inflammatory effects, including reduced neutrophil and monocyte cytokine production.11,31 DHA appears to be more responsible for the beneficial effects of fish and fish oils on lipids and lipoproteins, blood pressure, heart rate variability, and glycemic control, in comparison to EPA, while a mixture of DHA and EPA significantly reduced platelet aggregation in comparison to ALNA in vitro.11,32 The very-long chain n-3 polyunsaturated fatty acids powerfully lower serum triglycerides, but they raise LDL cholesterol.33 Therefore, their effect on coronary heart disease is probably mediated through pathways other than cholesterol.
Much of the epidemiologic evidence related to n-3 PUFAs is derived from the study of fish consumption in populations or interventions involving fish diets in clinical trials. Fish oils were, however, used in the GISSI study of 11 300 survivors of myocardial infarction.34 In this factorial design, fish oil (1 g/day) and vitamin E (300 mg/day) were compared, alone and in combination, to placebo. After 3.5 years of follow-up, the fish oil group had a statistically significant 20% reduction in total mortality, 30% reduction in cardiovascular death and 45% decrease in sudden death. While most published studies do not indicate that dietary n-3 PUFAs prevent restenosis after percutaneous coronary angioplasty or induce regression of coronary atherosclerosis, one study reported that occlusion of aortocoronary venous bypass grafts was reduced after 1 year by daily ingestion of 4g fish oil concentrate.35
The Lyon Diet Heart Study incorporated an n-3 fatty acid (alpha-linolenic acid) into a diet altered to develop a Mediterranean diet intervention.9 In the experimental group plasma ALNA and EPA increased significantly and the trial reported a 70% reduction in cardiovascular mortality at 5 years in its initial report. Total cholesterol and LDL cholesterol were identical in the experimental and control groups, suggesting that thrombotic and perhaps arrhythmic events may have been favorably influenced by n-3 PUFAs. Since the diet altered many other variables, such as fiber and antioxidants (by increasing fruit and vegetable consumption), direct attribution of benefits to n-3 PUFAs becomes difficult to establish.
The effect of different fatty acids on cardiac arhythmias has been an area of great interest. Diets rich in saturated fatty acids increase the risk of ventricular fibrillation and sudden cardiac death in primates. A population-based case – control study, using biomarkers, revealed a modest association of trans fatty acids in general and a strong association of trans isomers of linoleic acid in particular with primary cardiac arrest in humans.36 Several studies in different animal models, primate and rodent, have shown that n-3 PUFAs are protective against cardiac arrhythmias, especially ventricular fibrillation.37 It has been suggested that the fall in CHD mortality in the USA and Australia since 1967 is probably attributable to an increase in poly-unsaturate fat consumption in both countries since 1960.38 The decline in CHD mortality in the Zutphen cohort has similarly been attributed to a decreased consumption, over time, of trans fatty acids.28
The proportions of SFAs, MUFAs and PFAs as constituents of total fat intake and total energy consumption have engaged active attention, in view of the strong relationship of these fatty acids to the risk of CVD, especially CHD. The reduction of SFAs in the diet has been widely recommended, but its replacement remains an area of debate as to whether the place of reduced SFAs should be taken by MUFAs, PUFAs or carbohydrate. In a meta-analysis of studies on the effect of various dietary fats on blood lipids, the negative effect of dietary trans fat was about twice that of saturated fat on a calorie-for-calorie basis.39 Dietary trans fats have additional adverse effects, including increased serum triglyceride and lipoprotein (a) levels and adverse effects on endothelial function.40,41 Both MUFA and PUFA improve the lipoproteins profile, although polyunsaturated fatty acids are somewhat more effective. In view of this, recent US dietary recommendations suggested that SFAs should be reduced to 7 – 8%, MUFAs should be increased to 13 – 15% and PUFAs raised to 7 – 10% of daily energy, with the total fat contributing no more than 30% of all calories consumed.30,42 These may need to be adjusted for populations who consume lower quantities of total fat, so as to ensure an adequate intake of MUFAs and PUFAs even under those circumstances.
The total quantity of fat consumed, as a proportion of daily energy intake, has not shown a relation to CVD that is independent of the SFAs content. It has been argued that the type of fats consumed is far more important than the total amount of fat consumed.43 The compatibility of high-fat Mediterranean diets (with total fat contributing >30% of calories) with coronary protection has also been cited as supportive evidence. While the emphasis on the type of fat is well placed, it must be recognized that high-fat diets are also high in energy. Whether this contributes substantially to overweight is a subject of much debate.44 The Strong Heart Study examined the association between dietary fat intake and CHD incidence in American Indians.45 Participants (n = 2938) were followed for a mean (± SD) of 7.2 ± 2.3 years. In the age group of 47 – 59 years, those in the highest quartile of intake of total fat, saturated fatty acids or monounsaturated fatty acids had higher CHD mortality than did those in the lowest quartile (hazard ratio (95% CI): 3.57 (1.21–10.49), 5.17 (1.64–16.36), and 3.43 (1.17–10.04), respectively) after confounders were controlled for. These associations were not observed for those aged 60 – 79 years. Based on these strong independent risk associations, it would be prudent to reduce fat intake early in life to reduce the risk of dying from CHD.
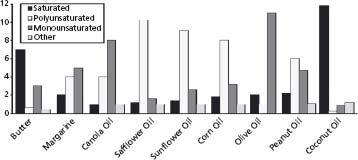
Enhancing the nutritional quality of dietary fat consumption, to provide greater cardiovascular protection, may be attempted by decreasing the sources of saturated fats and eliminating trans fatty acids in the diet, increasing the consumption of foods containing unsaturated fatty acids (both MUFAs and PUFAs) and decreasing dietary cholesterol consumption. Figure 23.2 provides a breakdown of different types of fats in the commonly consumed edible fats/oils.46
Figure 23.2 Breakdown of fat types in various fats and oils. (Reproduced with permission from Steckel46.)
Carbohydrates
The relationship of dietary carbohydrates to CVD appears to be mediated through indirect mechanisms: contribution to total energy and its effect on overweight and obesity; influence on central obesity; effects on plasma lipids, especially triglycerides, and effects on glycemic control. The balance between carbohydrates and fat as sources of energy as well as the fiber component of the diet are also areas of interest when considering this relationship. In feeding experiments, an increase in dietary energy from carbohydrates is usually associated with a moderate increase in fasting plasma triglyceride levels in the first few weeks but these return to near original levels eventually. Epidemiologically, high carbohydrate intakes are associated with low plasma cholesterol and variable plasma triglyceride concentrations.47
High-carbohydrate diets appear to reduce HDL cholesterol levels and increase the fraction of small dense LDL, both of which may impact adversely on vascular disease. This dyslipidemic pattern is consistent with the elevation of plasma triglycerides. There is as yet no clear evidence that the risk of CVD is altered independently by the carbohydrate levels in the diet. The glycemic index of foods might also be a determinant of the extent to which carbohydrates can influence the glycemic status. Carbohydrate diets with high glycemic index might adversely impact on glucose control, with associated changes in plasma lipids.48,49
Liu et al50 first reported a positive association between a higher dietary glycemic load (GL) and risk of CHD in the Nurses’ Health Study (NHS). Buelens et al51 report a similar association between GL and risk of CVD in the EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. The analysis included 15 714 women aged 49 – 70 years without diabetes or CVD at baseline. During 9 years of follow-up, 556 incident cases of major CVD events were documented. After adjusting for CVD risk factors and dietary fat and fiber, the investigators found a significant association between dietary GL and increased risk of CVD (risk ratio comparing extreme quartiles = 1.47, 95% confidence interval (CI) 1.04 – 2.09, P for trend = 0.03). In both the NHS and EPIC cohorts, the increased risk was more pronounced among overweight and obese women compared with normal-weight women.
It is mainly the amount and the type of carbohydrate consumed that will increase a person’s risk of developing cardiovascular disease, increased weight or type 2 diabetes. Non-refined carbohydrates, rich in dietary fiber, like wholegrains, fruit, vegetables and legumes, reduce the risk of heart disease. The greater risk of heart disease is associated with eating patterns high in refined carbohydrates, such as confectionery, cakes, biscuits, pastries and high-sugar drinks and fruit juice drinks. Limiting the amount of carbohydrate from foods containing refined carbohydrates is important for weight management and thus lowering the risk for heart disease.
Dietary fiber
Dietary fiber is a heterogeneous mixture of polysaccharides and lignin that cannot be degraded by the endogenous enzymes of vertebrate animals.53 Water-soluble fibers include pectins, gums, mucilages and some hemicelluloses. Insoluble fibers include cellulose and other hemicelluloses. Most soluble fibers reduce plasma total and LDL cholesterol concentrations, as reported by several trials.54 Pectins, psyllium, gums, mucilages, algal polysaccharides and some hemicelluloses lower total and LDL cholesterol levels without affecting HDL cholesterol, the reductions in total cholesterol being usually in the range of 5 – 10%. Human experiments have clearly shown that oat fiber tends to lower plasma total and LDL cholesterol but wheat fiber does not. Rice bran and barley may also lower cholesterol.55 Fiber consumption predicted insulin levels, weight gain and cardiovascular risk factors like blood pressure, plasma triglycerides, LDL and HDL cholesterol and fibrinogen more strongly than other dietary components in the CARDIA cohort study of young adults.56 However, fiber intake may be confounded with many other determinants of cardiovascular health.
BOX 23.1 Recommendations by the National Heart Foundation, Australia52
There is good evidence in the scientific literature to indicate that:
- there is no significant association between the total carbohydrate intake and the risk of developing cardiovascular disease
- eating patterns with a high glycemic load raise blood triglyceride levels, increasing the risk of cardiovascular disease
- eating patterns high in soluble fiber lower blood LDL cholesterol levels, reducing the risk of CVD
- total carbohydrate intake has no effect on insulin sensitivity or the risk of developing type 2 diabetes
There is moderate evidence in the scientific literature to indicate that:
- consuming dietary fiber from cereals (particularly wholegrains) and fruit is associated with a lower risk of cardiovascular disease
- eating patterns low in refined carbohydrates are associated with a lower risk of developing type 2 diabetes
Between 1996 and 2001, five very large cohort studies in the USA, Finland and Norway all reported that subjects consuming relatively large amounts of wholegrain cereals have significantly lower rates of CHD.55,57 High intake of fiber from cereal sources was associated with a reduced risk of CHD in the Nurses’ Health Study57 and was inversely associated with the risk of hypertension in the Health Professionals Follow-up Study.58
There are no randomized controlled trials evaluating the effects of wholegrain consumption on CHD events. However, numerous reviews have examined wholegrain intakes and the risk of CHD.59–62 Others have linked wholegrain consumption to lowered risk of diabetes and obesity in men63 and women.64 Both diabetes and overweight are risk factors for early-onset CHD and there is the possibility that modifying their risk could mediate some of the protective effects of wholegrains.60 Lowering of plasma total and LDL cholesterol has been shown in male and female Hispanic Americans.65 In contrast, Davy et al66 did not find a significant reduction in total or LDL cholesterol but demonstrated a lowering of more atherogenic small, dense LDL particles by oat bran relative to wheat cereal in sedentary, overweight American men.
Thus, the large body of prospective data provides convincing evidence for a protective effect of wholegrains against CHD. Available evidence supports a recommendation for consumption of about 15g /1000kcal of fiber.54 Since some of the reported benefits may have arisen from other dietary components occurring in association with fiber in natural foods, dietary consumption of high-fiber foods should be recommended rather than isolated fiber. Addition of oat bran may be considered, where necessary, to supplement natural foods in order to attain the recommended dietary intake.
Antioxidants
The oxidation of LDL by oxygen free radicals results in the unregulated uptake of modified LDL by macrophages in arterial walls, accelerating the atherosclerotic process. Anti-oxidant nutrients, which can directly scavenge free radicals, include alpha-tocopherol (vitamin E isomer) and ascorbic acid (vitamin C), which have shown antioxidant activity both in vitro and in vivo, as well as beta-carotene (a provitamin A carotenoid) which has displayed antioxidant activity in vitro.67 These mechanisms suggested that increased dietary intake or supplementation of these nutrients would be protective against atheroslerotic vascular disorders. This was supported by evidence from observational studies for vitamin E and beta-carotene, but results of clinical trials employing supplements have been disappointing.
Observational cohort data suggest a protective role for carotenoids. The randomized trials, in contrast, reported a moderate adverse effect of beta-carotene supplementation, with a relative increase in the risk of cardiovascular death of 12% in a meta-analysis of four trials.68 Cancer risk was also increased.
Several large cohort studies showed significant reductions in the incidence of cardiac events in men and women taking high-dose vitamin E supplements.67 However, the HOPE trial, a definitive clinical trial relating vitamin E supplementation to cardiovascular outcomes, revealed no effect of vitamin E supplementation (at 400 IU/day, for a mean follow-up of 4.5 years) on MI, stroke or death from cardiovascular causes in men or women.69 Other trials also failed to demonstrate a cardioprotective effect of vitamin E supplements.70 A recent meta-analysis also showed no evidence of a protective effect of antioxidant or B vitamin supplements on the progression of atherosclerosis.71
Flavonoids are polyphenolic antioxidants which occur in a variety of foods of vegetable origin, such as tea, onions and apples. Data from several prospective studies indicate an inverse association of dietary flavonoids with coronary heart disease.65 A benefit on stroke risk has also been reported.66 However, confounding may be a major problem and may explain conflicting results of observational studies on flavonoids and coronary heart disease. Fruits and vegetables also contain other phytochemicals that may have protective properties, including isothiocyanates and indoles (found in cruciferous vegetables), sulfides (found in onions and garlic), terpenes (found in citrus oils) and phytoestrogens.54 The overall health benefit of flavonoid consumption is unproven, and their intake in the form of fortified foods or supplements should not be encouraged.72
Systematic reviews73,74 appraising large amounts of epidemiologic and observational evidence suggest that flavonoids in chocolates may lower the risk of CHD mortality. Crude approximations suggest that eating 50 g of dark chocolate per day may reduce one’s risk of CVD by 10.5% (95% CI 7.0 – 13.5%). However, these estimates were based on results from studies of short duration, extrapolated to long-term CVD outcomes. Long-term randomized feeding trials, beyond short-term studies of CVD risk factor intermediates, are warranted to investigate the impact of chocolate consumption on cardiovascular outcomes.
Phenolics in red wine were shown to be able to inhibit the oxidation of LDL in vitro and this was suggested as an explanation of the “French paradox”.75 The Zutphen study, an epidemiologic study in The Netherlands, suggested an inverse correlation between the incidence of CHD and stroke and the dietary intake of flavonoids, especially quercetin.76
Green tea polyphenols have been studied extensively as CVD chemopreventive agents. A population-based prospective cohort study (the Ohsaki Study) examined the association between green tea consumption and mortality from CVD, cancer and all causes with 40 530 persons in Miyagi Prefecture, in northern Japan.77 Green tea consumption was inversely associated with mortality from CVD and this inverse association was more pronounced in women (P = 0.08 for interaction with sex). Kuriyama78 reviewed the association between green tea or green tea extracts and CVD risk profiles and reported that more than half of the trials have demonstrated the beneficial effects of green tea on CVD risk profiles. These results suggest a plausible mechanism for the beneficial effects of green tea.
Sesso et al79 determined whether the intake of lycopene or tomato-based foods is associated with the risk of CVD in a prospective cohort of 39 876 middle-aged and older women initially free of CVD and cancer. They found that the dietary lycopene was not strongly associated with the risk of CVD. However, some inverse associations for CVD, noted for higher levels of tomato-based products, particularly tomato sauce and pizza, suggested that dietary lycopene or other phytochemicals consumed as oil-based tomato products could confer cardiovascular benefits.
The conflict between diet-based observational studies and clinical trials employing supplements may arise because of one or more explanatory factors: confounding, interactions/synergistic activity (among antioxidants; with other nutrients), isomers with differing activity in food compared to supplements, other associated protective elements in natural foods (e.g. flavonoids, phytoestrogens) and/or temporal dissociation of antioxidant blood levels from fat intake in meals, when administered as once-daily pills. While the failure of pill supplementation does not necessarily exclude protective effects of dietary antioxidants, current evidence does not support supplementation of any of these antioxidant vitamins for prevention of CHD. However, intake of their primary food resources, especially fruit and vegetables, may be encouraged.
Folate
The relationship of folate to CVD has been mostly explored through its effect on homocysteine (HCY), which has been incriminated as an independent risk factor for CHD and probably stroke.80–83 Folic acid is required for the methylation of homocysteine to methionine. Reduced plasma folate has been strongly associated with elevated plasma homocysteine levels and folate supplementation has been demonstrated to decrease those levels.84 However, the role of homocysteine as an independent risk factor for CVD has been subject to debate, in view of the data from several prospective studies which did not find this association to be independent of other risk factors.85 Furthermore, clinical trials such as the Norwegian Vitamin Trial (NORVIT),86 Vitamin Intervention for Stroke Prevention (VISP)87 and HOPE-288 showed that, even though vitamin supplementation reduced HCY levels, there was no significant effect on cardiovascular risk. It has also been suggested that elevation of plasma HCY is a consequence and not a cause of atherosclerosis, wherein impaired renal function due to atherosclerosis raises plasma HCY levels.89 At present, we cannot recommend the use of vitamin supplementation to reduce CVD risk.
Minerals: blood pressure and cardiovascular disease
Sodium
High blood pressure (HBP) is a major risk factor for CHD and both forms of stroke (ischemic and hemorrhagic). The relative risk of CVD for both systolic and diastolic blood pressures operates in a continuum of increasing risk for rising pressure but the absolute risk of CVD is considerably modified by co-existing risk factors.90 Of the many risk factors associated with high blood pressure, the dietary exposure most investigated has been daily sodium consumption. It has been studied extensively in animal experimental models, in epidemiologic studies, controlled clinical trials and in population studies on restricted sodium intake.90 Salt or sodium intake has been directly correlated with mean blood pressure levels and prevalence of hypertension in many populations. Comprehensive epidemiologic evidence was provided by the INTERSALT Study91,92 which investigated the relationship of 24-hour urinary electrolyte excretion to blood pressure in 52 population groups across 32 countries, using standardized methodology to provide comparable data. In adults aged 20 – 59 years, there was a significant positive relationship between urinary sodium excretion and blood pressure across the 52 population samples. Further, it was also observed that in four of these populations in whom the mean 24-hour urinary sodium excretion was lower than 100 mmol/day, systolic blood pressure did not rise with age.93
The effects on blood pressure levels of increased sodium consumption accompanying urbanization were demonstrated in the Kenyan Luo Migration Study wherein rural farmers who traditionally consumed a low-salt diet were observed to have an elevation of blood pressure when they migrated to an urban environment. These migrants exhibited blood pressure levels higher than rural controls and comparable to levels observed in Western populations.94 This rise in blood pressure was related to an increase in salt consumption and a reduced dietary intake of potassium. An overview of observational data in populations suggested that a difference in sodium intake of 100 mmol/day could be associated with average differences in systolic blood pressure of 5 mmHg at age 15 – 19 years and 10 mmHg at 60–69 years.95 Diastolic blood pressures are reduced by about half as much, but the association increases with age and the magnitude of the initial blood pressure. It was estimated that a universal reduction in dietary intake of salt by 50 mmol/day would lead to a 50% reduction in the number of people requiring antihypertensive therapy, a 22% reduction in number of deaths due to strokes and a 16% reduction in number of deaths from coronary heart disease.95
A cohort study in Finland prospectively followed 1173 men and 1263 women aged 25 – 64 years, with complete data on 24-hour urinary sodium excretion and cardiovascular risk factors.96 The hazard ratios for CHD, CVD and all-cause mortality, associated with a 100 mmol increase in 24-hour urinary sodium excretion, were 1.51 (95% CI 1.14 – 2.00), 1.45 (1.14 – 1.84) and 1.26 (1.06 – 1.50) respectively, in both men and women. The frequency of acute coronary events, but not acute stroke events, rose significantly with increasing sodium excretion. Disaggregated analyses revealed significant risk ratios in men only and revealed that sodium predicted mortality in men who were overweight. Despite the limitations of such subgroup analyses, the overall association of increasing sodium excretion with CVD and all-cause mortality further supports the evidence linking increased sodium intake to adverse cardiovascular health outcomes.
Several clinical intervention trials, conducted to evaluate the effects of dietary salt reduction on blood pressure levels in hypertensive and normotensive individuals, have been systematically reviewed.12,97 Many of the earlier trials were of limited size and short duration and were deficient in statistical power. Based on an overview of 32 methodologically adequate trials (22 in hypertensive subjects and 12 in normotensive persons), Cutler et al12 concluded that a daily reduction in intake of sodium by 70 – 80 mmol was associated with a lowering of blood pressure in both hypertensive and normotensive individuals, with systolic and diastolic blood pressure reductions of 4.8/1.9 mmHg in the former and 2.5/1.1 mmHg in the latter. Clinical trials have also demonstrated the sustained blood pressure lowering effects of sodium restriction in infancy,98 as well as in the elderly in whom it provides a useful non-pharmacologic therapy.99 In a 3-year study of salt restriction by Cook et al,100 the Trials of Hypertension Prevention (TOHP), Phase II, participants had a decrease in systolic blood pressure (1.3mmHg, P = 0.02) that corresponded with a significant dose-dependent reduction in sodium excretion. The Trial of Nonpharmacologic Interventions in the Elderly (TONE) was the first multicenter clinical trial of sufficient size and duration to show that lifestyle modifications can be used to control high blood pressure in older people. In elderly participants, sodium restriction resulted in mean reductions of 4.3 mmHg (P < 0.001) and 2.0mmHg (P = 0.001) in systolic and diastolic blood pressure, respectively.101
The effect of a multi-component lifestyle intervention that includes the combination of sodium restriction, the DASH diet, weight loss and regular aerobic exercise was evaluated in the Diet, Exercise, and Weight Loss Intervention Trial (DEW-IT).102 After 9 weeks, systolic and diastolic blood pressures were decreased by 12.1 mmHg (P < 0.001) and 6.6mmHg (P < 0.001) respectively in the intervention participants compared with those in the control group. In subjects with above optimal blood pressure, the PREMIER trial showed that those in the “established recommendations” or “established + DASH diet” intervention groups had significant weight loss and reduction in sodium intake; both groups achieved greater reductions in systolic and diastolic blood pressure than did patients in the “advice only” group.103
The results of the low sodium – DASH diet trial104
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


