DIAGNOSTIC CARDIAC CATHETERIZATION AND CORONARY ANGIOGRAPHY
Diagnostic cardiac catheterization and coronary angiography are considered the gold standard in the assessment of the anatomy and physiology of the heart and its associated vasculature. In 1929, Forssmann demonstrated the feasibility of cardiac catheterization in humans when he passed a urological catheter from a vein in his arm to his right atrium and documented the catheter’s position in the heart by x-ray. In the 1940s, Cournand and Richards applied this technique to patients with cardiovascular disease to evaluate cardiac function. These three physicians were awarded the Nobel Prize in 1956. In 1958, Sones inadvertently performed the first selective coronary angiography when a catheter in the left ventricle slipped back across the aortic valve, engaged the right coronary artery, and power-injected 40 mL of contrast down the vessel. The resulting angiogram provided superb anatomic detail of the artery, and the patient suffered no adverse effects. Sones went on to develop selective coronary catheters, which were modified further by Judkins, who developed preformed catheters and allowed coronary artery angiography to gain widespread use as a diagnostic tool. In the United States, cardiac catheterization is the second most common operative procedure, with nearly 3 million procedures performed annually.
CARDIAC CATHETERIZATION
INDICATIONS, RISKS, AND PREPROCEDURE MANAGEMENT
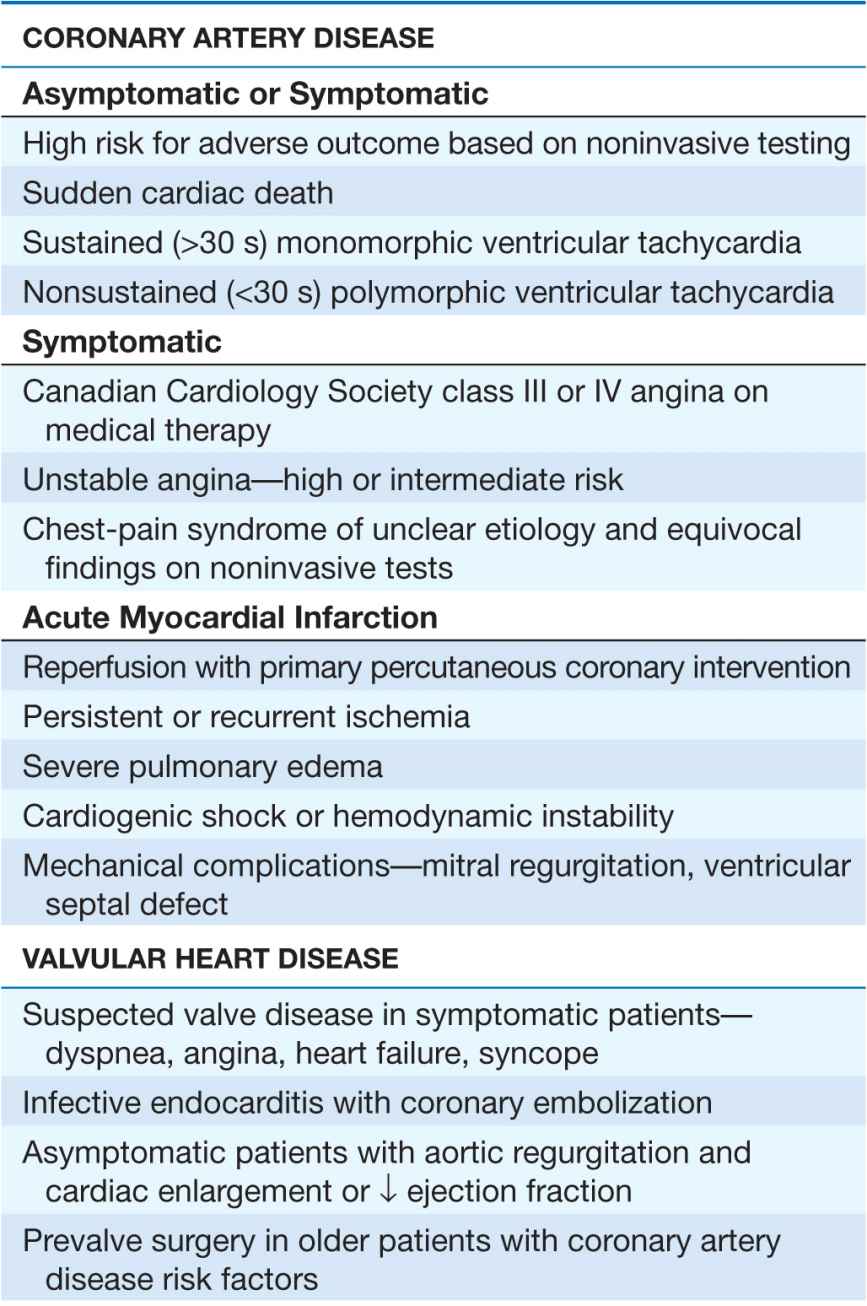
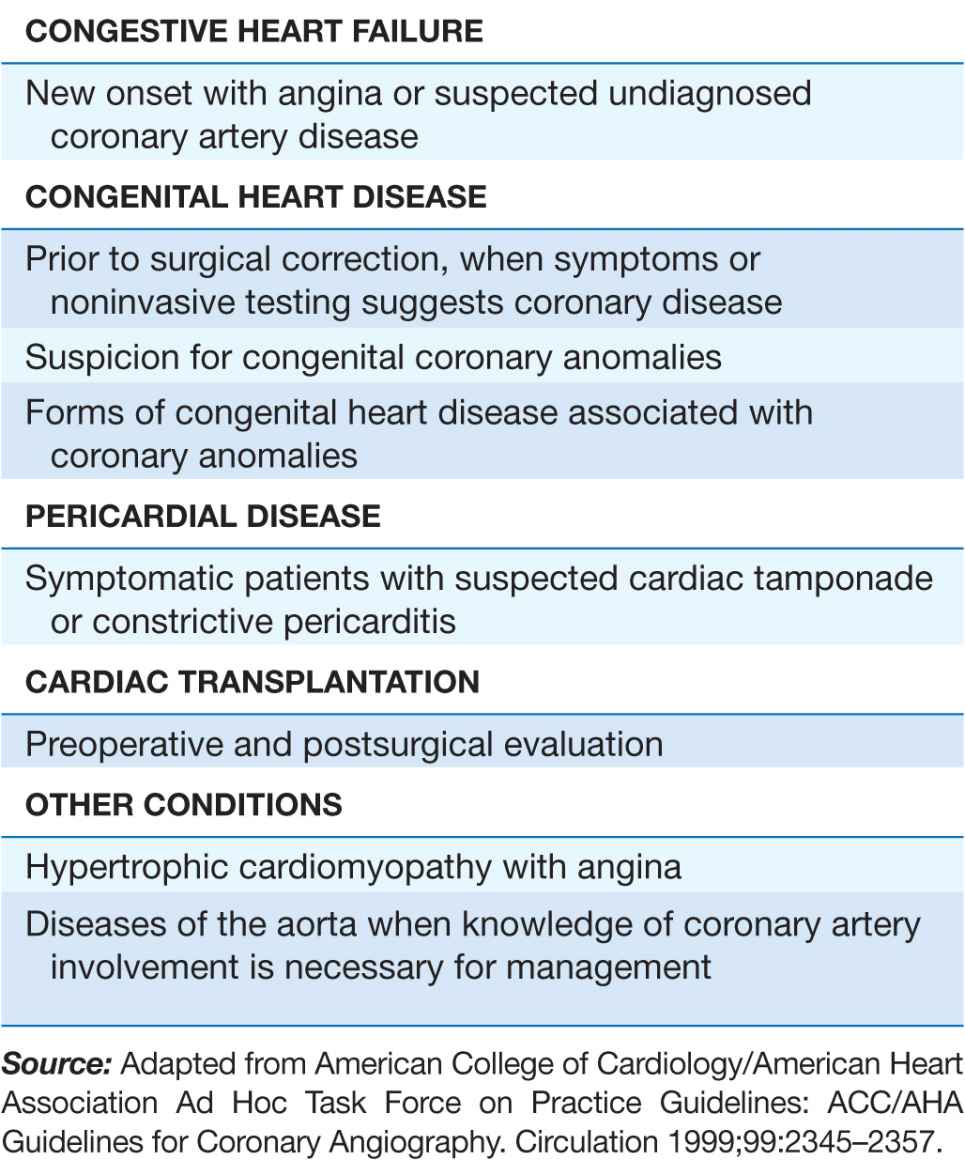
Cardiac catheterization and coronary angiography are indicated to evaluate the extent and severity of cardiac disease in symptomatic patients and to determine if medical, surgical, or catheter-based interventions are warranted (Table 13-1). They are also used to exclude severe disease in symptomatic patients with equivocal findings on noninvasive studies and in patients with chest-pain syndromes of unclear etiology for whom a definitive diagnosis is necessary for management. Cardiac catheterization is not mandatory prior to cardiac surgery in some younger patients who have congenital or valvular heart disease that is well defined by noninvasive imaging and who do not have symptoms or risk factors that suggest concomitant coronary artery disease.
TABLE 13-1
INDICATIONS FOR CARDIAC CATHETERIZATION AND CORONARY ANGIOGRAPHY
The risks associated with elective cardiac catheterization are relatively low, with a reported risk of 0.05% for myocardial infarction, 0.07% for stroke, and 0.08–0.14% for death. These risks increase substantially if the catheterization is performed emergently, during acute myocardial infarction, or in hemodynamically unstable patients. Additional risks of the procedure include tachy- or bradyarrhythmias that require countershock or pharmacologic therapy, acute renal failure leading to transient or permanent dialysis, vascular complications that necessitate surgical repair, and significant access-site bleeding. Of these risks, vascular access-site bleeding is the most common complication, occurring in 1.5–2.0% of patients, with major bleeding events associated with a worse short- and long-term outcome.
In patients who understand and accept the risks associated with cardiac catheterization, there are no absolute contraindications when the procedure is performed in anticipation of a life-saving intervention. Relative contraindications do, however, exist; these include decompensated congestive heart failure; acute renal failure; severe chronic renal insufficiency, unless dialysis is planned; bacteremia; acute stroke; active gastrointestinal bleeding; severe, uncorrected electrolyte abnormalities; a history of an anaphylactic/anaphylactoid reaction to iodinated contrast agents; and a history of allergy/bronchospasm to aspirin in patients for whom progression to a percutaneous coronary intervention is likely.
Contrast allergy and contrast-induced renal failure merit further consideration, because these adverse events may occur in otherwise healthy individuals and prophylactic measures exist to reduce risk. Allergic reactions to contrast agents occur in <5% of cases with severe anaphylactoid (clinically indistinguishable from anaphylaxis, but not mediated by an IgE mechanism) reactions occurring in 0.1–0.2% of patients. Mild reactions manifest as nausea, vomiting, and urticaria, while severe anaphylactoid reactions lead to hypotensive shock, pulmonary edema, and cardiorespiratory arrest. Patients with a history of significant contrast allergy should be premedicated with corticosteroids and anti-histamines (H1– and H2-blockers) and studies performed with nonionic, low-osmolar contrast agents that have a lower reported rate of allergic reactions.
Contrast-induced nephropathy, defined as an increase in creatinine >0.5 mg/dL or 25% above baseline that occurs 48–72 h after contrast administration, occurs in ~2–7% of patients with rates of 20–30% reported in high-risk patients, including those with diabetes mellitus, congestive heart failure, chronic kidney disease, anemia, and older age. Dialysis is required in 0.3–0.7% of patients and is associated with a five-fold increase in in-hospital mortality. For all patients, adequate intravascular volume expansion with intravenous 0.9% saline (1.0–1.5 mL/kg per hour) for 3–12 h before and continued 6–24 h after the procedure limits the risk of contrast-induced nephropathy. In patients with chronic kidney disease, additional pretreatment with N-acetylcysteine (Mucomist, 600 mg bid orally before and two days after catheterization) also decreases risk. Diabetic patients treated with metformin should stop the drug 48 h prior to the procedure to limit the associated risk of lactic acidosis. Other strategies to decrease risk include the administration of sodium bicarbonate, although there is conflicting data regarding its efficacy; use of low- or iso-osmolar contrast agents; and limiting the volume of contrast to <100 mL per procedure.
Cardiac catheterization is performed after the patient has fasted for 6 h and has received IV conscious sedation to remain awake but sedated during the procedure. All patients with suspected coronary artery disease are pretreated with 325 mg aspirin. In patients in whom the procedure is likely to progress to a percutaneous coronary intervention, a clopidogrel 600-mg loading dose followed by 75 mg daily should be started. Warfarin is held starting 48 h prior to the catheterization to allow the international normalized ratio (INR) to fall to <2.0 and limit access-site bleeding complications. Cardiac catheterization is a sterile procedure, so antibiotic prophylaxis is not required.
TECHNIQUE
Cardiac catheterization and coronary angiography provide a detailed hemodynamic and anatomic assessment of the heart and coronary arteries. The selection of procedures is dependent upon the patient’s symptoms and clinical condition, with some direction provided by noninvasive studies.
Vascular access
Cardiac catheterization procedures are performed using a percutaneous technique to enter the femoral artery and vein as the preferred access sites for left and right heart catheterization, respectively. A flexible sheath is inserted into the vessel over a guidewire, allowing diagnostic catheters to be introduced into the vessel and advanced toward the heart using fluoroscopic guidance. The brachial or radial artery may also be used as an arterial access site in patients with peripheral arterial disease that involves the abdominal aorta, iliac, or femoral vessels; severe iliac-artery tortuosity; morbid obesity; or preference for early postprocedure ambulation. Use of radial-artery access is gaining popularity owing to a lower rate of access-site bleeding complications. A normal Allen’s test confirming dual blood supply to the hand from the radial and ulnar arteries is a prerequisite to access this site. The internal jugular vein serves as an alternate access site to the right heart when the patient has an inferior vena cava filter in place or requires prolonged hemodynamic monitoring.
Right heart catheterization
This procedure measures pressures in the right heart. Right heart catheterization is no longer a routine part of diagnostic cardiac catheterization, but it is reasonable in patients with unexplained dyspnea, valvular heart disease, pericardial disease, right and/or left ventricular dysfunction, congenital heart disease, and suspected intracardiac shunts. Right heart catheterization uses a balloon-tipped flotation catheter that is inserted into the femoral or jugular vein. Using fluoroscopic guidance, the catheter is advanced sequentially to the right atrium, right ventricle, pulmonary artery, and pulmonary wedge position (as a surrogate for left atrial pressure); in each cardiac chamber, pressure is measured and blood samples are obtained for oxygen-saturation analysis to screen for intracardiac shunts.
Left heart catheterization
This procedure measures pressures in the left heart as a determinant of left ventricular performance. With the aid of fluoroscopy, a catheter is guided to the ascending aorta and across the aortic valve into the left ventricle to provide a direct measure of left ventricular pressure. In patients with a tilting-disc prosthetic aortic valve, crossing the valve with a catheter is contraindicated and the left heart may be accessed from the right atrium using a needle-tipped catheter to puncture the atrial septum at the fossa ovalis. Once the catheter crosses from the right to the left atrium, it can be advanced across the mitral valve to the left ventricle. This technique is also used for mitral valvuloplasty. Heparin is given for prolonged procedures to limit the risk of stroke from embolism of clots that may form on the catheter.
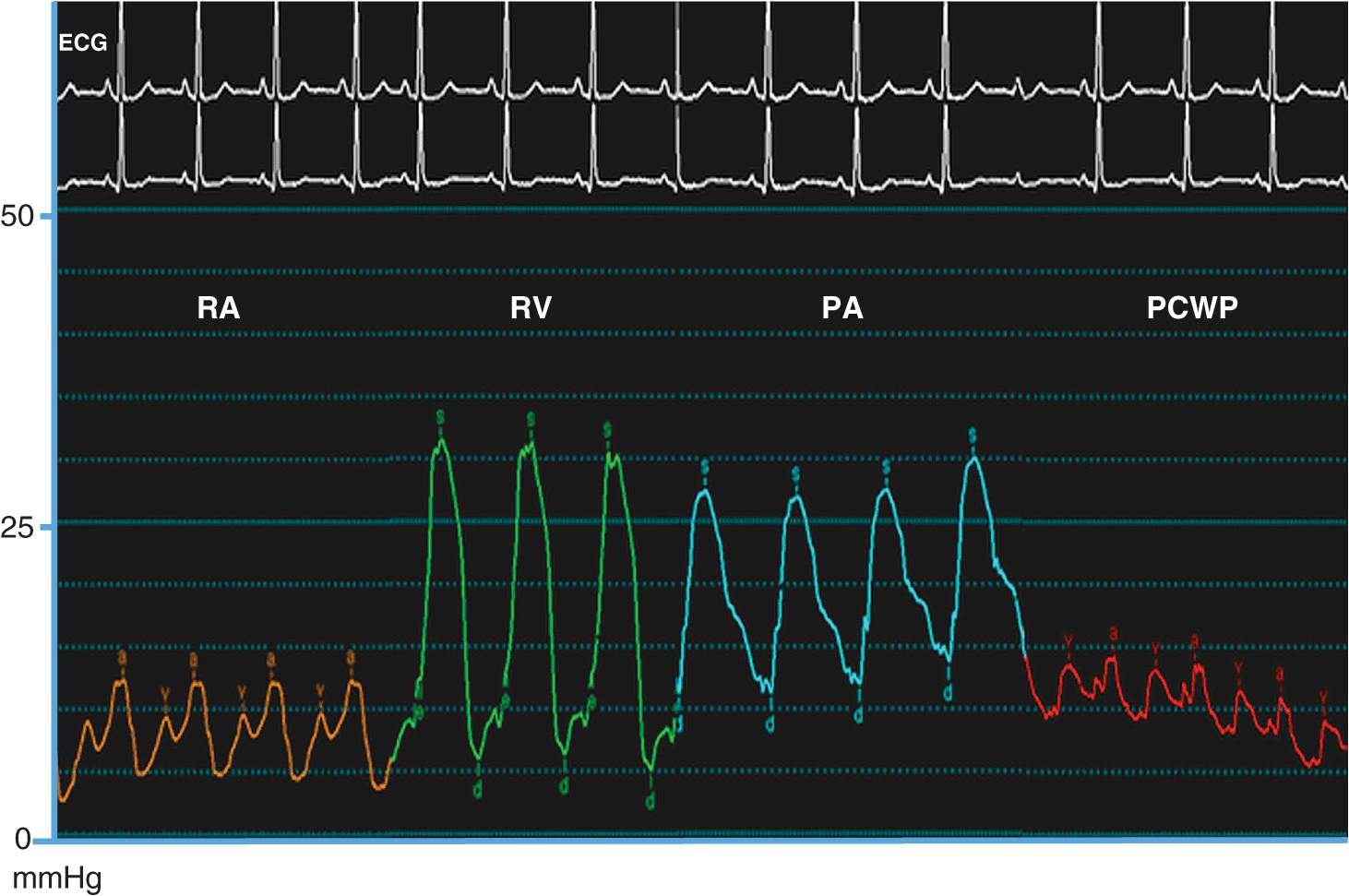
HEMODYNAMICS
A comprehensive hemodynamic assessment involves obtaining pressure measurements in the right and left heart and peripheral arterial system and determining the cardiac output (Table 13-2). The shape and magnitude of the pressure waveforms provide important diagnostic information; an example of normal pressure tracings is shown in Fig. 13-1. In the absence of valvular heart disease, the atria and ventricles are “one chamber” during diastole when the tricuspid and mitral valves are open while in systole, when the pulmonary and aortic valves are open, the ventricles and their respective outflow tracts are considered “one chamber.” These concepts form the basis by which hemodynamic measurements are used to assess valvular stenosis. When aortic stenosis is present, there is a systolic pressure gradient between the left ventricle and the aorta; when mitral stenosis is present, there is a diastolic pressure gradient between the pulmonary capillary wedge (left atrial) pressure and the left ventricle (Fig. 13-2). Hemodynamic measurements also discriminate between aortic stenosis and hypertrophic obstructive cardiomyopathy where the asymmetrically hypertrophied septum creates a dynamic intraventricular pressure gradient during ventricular systole. The magnitude of this obstruction is measured using an end-hole catheter positioned at the left ventricular apex that is pulled back while recording pressure; once the catheter has passed the septal obstruction and is positioned in the apex of the left ventricle, a gradient can be measured between the left ventricular apex and the aorta. Hypertrophic obstructive cardiomyopathy is confirmed by the Brockenbrough-Braunwald sign: following a premature ventricular contraction, there is an increase in the left ventricular–aorta pressure gradient with a simultaneous decrease in the aortic pulse pressure. These findings are absent in aortic stenosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree