Diagnosis and Management of Diseases of the Peripheral Arteries and Veins: Introduction
Peripheral vascular diseases are a diverse collection of disorders that affect all organ systems. Although peripheral arterial disease (PAD) is the disease most commonly encountered by the cardiologist, disease of the lymphatics and veins is equally common (globally more so). For the cardiologist or internist with an interest in vascular disorders, a systematic and comprehensive approach is required. This chapter covers commonly encountered areas of vascular disease including lymphedema, venous disease, and PAD. Accompanying chapters on aortic and cerebrovascular disease address those areas in more detail.
Lymphedema
Lymphedema is an abnormal buildup of lymphatic fluid in the dermal and subcutaneous tissues. In contrast to the venous system, the fragile and easily damaged lymphatic vessels carry a low volume of flow but do so actively, propelling flow from the periphery centrally via peristalsis. As vessels coalesce, larger and larger conduits form eventually becoming the thoracic duct that empties into the left subclavian vein.
Lymphedema may be primary or secondary in etiology. Primary lymphedema may be congenital (present at birth) or, more commonly, present in the early teen years (lymphedema praecox) and is more common in females presenting around menarche. Lymphedema tarda presents in later years and is a diagnosis of exclusion because a secondary cause of is much more likely in this age group. Trauma, recurrent infection, obstruction, infiltration, and radiation all cause lymphatic vessel damage. Lymph nodes are located along lymphatic vessels. Globally, lymphedema is the most common vascular disease, affecting 90 to 120 million people.1 Mosquito-borne infection with filaria is endemic in tropical countries. However, cases occur within the contiguous United States including “cold weather” states.2 Upper extremity lymphedema may occur after axillary node dissection. Recurrent cellulitis is common in patients with lymphedema and may be an initiating, exacerbating, or complicating event. Streptococcus is the most common organism, entering the skin through a crack in the toe webs caused by tinea pedis. The organism damages the lymphatic channels and the lymph nodes, with repeated infection eventually obliterating the vessel.3
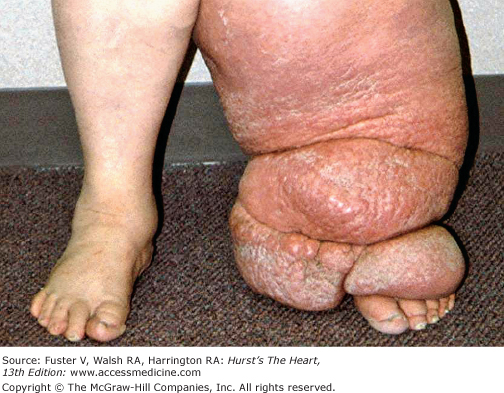
History and physical examination make the diagnosis in the majority of cases. Unlike edema and lipedema, lymphedema involves the toes and often affects them first. The skin is thickened and takes on an orange peel consistency (peau d’orange) (Fig. 109–1). A diffuse, flat, warty consistency may affect the skin over time. Dependent edema spares the toes unless secondary lymphedema is present. Lipedema is caused by excess fatty deposits in the leg and may be difficult to differentiate from lymphedema if the foot is not examined. However, with lipedema, the toes are spared, and there is often a ridge or fold overhanging the ankle.
The techniques currently available for imaging of the lymphatic system are lymphangiography and lymphoscintigraphy. Lymphangiog-raphy is difficult to perform and carries a risk of iatrogenic lymphangitis. Very few individuals now perform this test. The lymphoscintigram is based on uptake and ascent of technetium 99–labeled antimony trisulfide colloid after injection between the web spaces of digits. It is easier to perform than lymphangiography, has a low risk of lymphangitis, and has good ability to differentiate lymphedema from other causes of edema. It cannot reliably distinguish primary from secondary lymphedema.4,5 A dermal back flow pattern is typical in patients with lymphedema. Magnetic resonance imaging of the leg is also used to differentiate lipedema from edema or lymphedema, but functional data are not obtained.
Treatment for lymphedema is volume reduction of the limb. Reduction in limb size by elevation, mechanical pumping, or manual massage is effective. Wrapping of the limb, distal to proximal, is required whenever the patient is up. After leg volume is decreased, an elastic compression garment, 40 to 50 mm Hg in strength, should be worn daily, replaced two to four times per year as needed.6 Early and aggressive treatment of cellulitis and fungal infections of the toes helps to prevent cellulitis.
Laboratory Assessment of Venous Disease
Duplex ultrasound, computed tomography (CT), and magnetic resonance venography (MRV) are the methods available for evaluation of venous anatomy, but contrast venography is considered the gold standard.7 Venography must be performed by both ascending and descending approaches to completely evaluate the veins for obstruction and incompetence. For many reasons, venous duplex ultrasound is the most commonly used method. It has the advantage of differentiating acute from old thrombus based on the presence or absence of venous distension (common with acute clot) and increased echogenicity (common with chronic clot). Compared with venography, duplex ultrasound is less sensitive both above the groin and below the knee.8 MRV and CT venography are rapidly emerging technologies that are proving to be accurate and applicable in clinical practice. In addition to providing accurate information above the groin, both may be performed concurrently with pulmonary embolism studies.9,10 With regard to blood work, a negative D-dimer profile has proven to be an excellent test to exclude presence of acute thrombosis.11,12
Continuous-wave Doppler (CWD) provides qualitative information about blood flow. A loss of phasicity with respiration suggests venous obstruction. When a Doppler is placed over a vein and the limb distal to the probe is compressed, there should be augmentation of venous flow. If not, obstruction is present. Conversely, increased flow signal during proximal compression suggests venous valvular incompetence. By examining the limb at multiple levels, localization of incompetence or obstruction can be made (specificity, 88%; sensitivity, 85%).13 CWD alone is a poor technique for evaluating partially obstructing thrombus or confirming acute deep vein thrombosis (DVT). For this reason, Duplex ultrasound with Doppler evaluation of the limb is used much more commonly.14
Plethysmography measures the volume change in the limb (due to arterial inflow, venous outflow, or venous reflux) over time. The most common plethysmographic techniques are strain gauge plethysmography, air plethysmography, and impedance plethysmography (IPG). Outflow plethysmography is useful in screening for venous outflow obstruction.15 IPG is the best-studied technique. Unlike venography or duplex ultrasound, IPG identifies the presence of functional rather than anatomic venous obstruction.16 IPG screening has been replaced by Duplex ultrasound in most centers. Venous insufficiency is assessed by raising the legs to attain an empty state and then rapidly lowering the legs. If incompetent, blood rushes from the proximal veins (retrograde) into the calf and volume increases rapidly.17 If incompetence is present in the superficial veins only, placing light tourniquets around the leg or directly compressing an incompetent superficial vein will normalize refilling time. Exercise plethysmography assesses ejection of blood by calf pump function and the subsequent refilling of the lower extremities.
Clinical Venous Disease
Venous disease is common, with cross-sectional studies demonstrating some form of venous disease present in >50% of the population of Edinburgh Scotland.18-20 Venous disease is extremely variable with multiple clinical presentations and causes. The CEAP classification organizes this by the clinical manifestations, etiologic factors, anatomic involvement, pathophysiologic features, but the clinical classification is commonly used alone21,22 (Table 109–1).
| Clinical Presentation | Etiology | Anatomyb | Pathophysiology | |
|---|---|---|---|---|
| C0 | No venous disease | EC congenital | AS superficial veins | PR reflux |
| C1 | Telangiectasia, or reticular veins | |||
| C2 | Varicose veins | EP primary | AD deep veins | PO obstruction |
| C3 | Edema without skin changes | |||
| C4a | Skin pigmentation or eczema | ES secondary | AP perforator veins | PR,O reflux and obstruction |
| C4b | Lipodermatosclerosis or atrophie blanche | |||
| C5 | Healed ulceration | |||
| C6 | Active ulceration |
Primary varicosities tend to be familial and without other causative events. They often first appear during pregnancy. Prolonged dependency at the place of work may increase the risk of developing varicose veins.23 Secondary varicosities may be caused by several etiologies including extrinsic venous compression, prior DVT, congenital lesions, arteriovenous fistulas, right heart disease, or perforator vein incompetence. History, examination, and laboratory evaluation of the deep venous system allow differentiation of primary from secondary varicosities.
“Burning,” “bursting,” “bruised,” and “aching” are just some of the sensations reported by patients.24 Symptoms are exacerbated by prolonged standing or dependency, during pregnancy, or by other volume overload states. Elevation relieves symptoms. If the discomfort worsens with elevation, it is unlikely due to varicosities. Episodes of superficial thrombophlebitis may occur, and if repetitive, ablation of the veins is appropriate. Symptoms and progression can be improved by graduated compression hose. Ablation of the vein should be considered if complications or discomfort interfere with occupation or lifestyle.25 Sclerotherapy is effective for small varicosities and larger spider veins. Laser therapy is effective for small spider veins and telangectasias.26 Traditionally, surgical removal was indicated for long segments and proximal varicosities, especially if perforator vein or saphenofemoral junction incompetence was present.27 Currently, endovascular techniques are favored, including laser ablation and radiofrequency ablation, often as outpatient procedures.28
Superficial thrombophlebitis (STP) presents as a tender, erythematous, indurated lesion following the course of a superficial vein that, on palpation, feels like a cord. STP often occurs in varicose veins or at sites of indwelling catheters or recent intravenous injections. Cellulitis is common, and when present, antibiotics should be used. STP is usually self-limited, and recovery may be accelerated by rest, elevation, warm compresses, and anti-inflammatory agents. There is a moderate incidence of concurrent DVT in STP.29 Duplex ultrasound should be used to screen for DVT when clinically suspected because management with chronic anticoagulation is then indicated. Systemic anticoagulation is appropriate when phlebitis progresses despite conservative care or when located very near the deep venous system (ie, within 1-2 cm of the saphenofemoral junction) and minimal extension would enter the deep system.30 Evaluation for underlying thrombophili atesting for a clotting abnormality should be considered in the setting of recurrent STP or in those with a strong family history of thrombosis.31
The morbidity and mortality of DVT are high. Risk factors for DVT and pulmonary embolism have been well defined in several studies32,33 (Table 109–2). The signs and symptoms of DVT are nonspecific and unreliable.34 Objective testing to confirm and define the extent of DVT should be obtained whenever the diagnosis is entertained.35 If the DVT is not present, the cost and risks of treatment, including hemorrhage, heparin-induced thrombocytopenia, and warfarin necrosis, are avoided.
| Transient Cause | Fixed Cause |
|---|---|
| Recent surgery | Prior superficial vein thrombosis |
| Hospitalization | Prior deep venous thrombosis |
| Trauma | Residence in health care facility |
| Malignancy | Immobility |
| Hormonal therapy | Age |
Treatment with heparin acutely and warfarin chronically is highly effective in preventing clot propagation and pulmonary embolism. Low molecular weight heparins (adjusted for weight) have proven effective in treating DVT and can be used for outpatient management in uncomplicated cases.36 Heparin-induced thrombocytopenia is common; platelets should be monitored routinely while on heparin. Heparin-induced thrombocytopenia is confirmed by detecting platelet factor 4 antibodies.37 When present, a direct thrombin inhibitor or danaparoid is used in place of heparin until the warfarin effect is therapeutic.38 Warfarin-induced necrosis, although rare, may be avoided by overlapping heparin with warfarin for 4 to 5 days.39
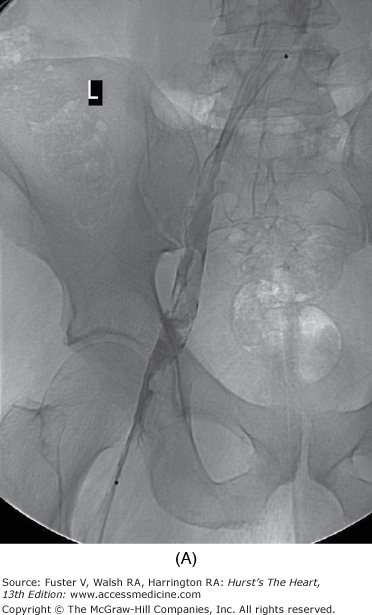
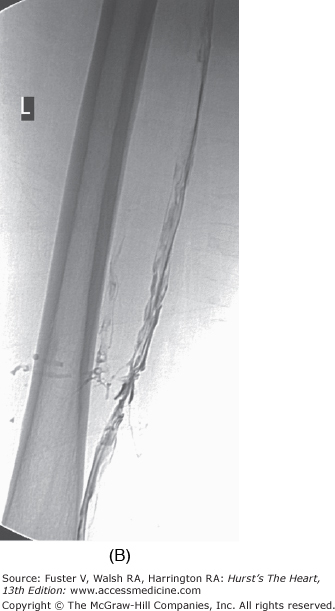
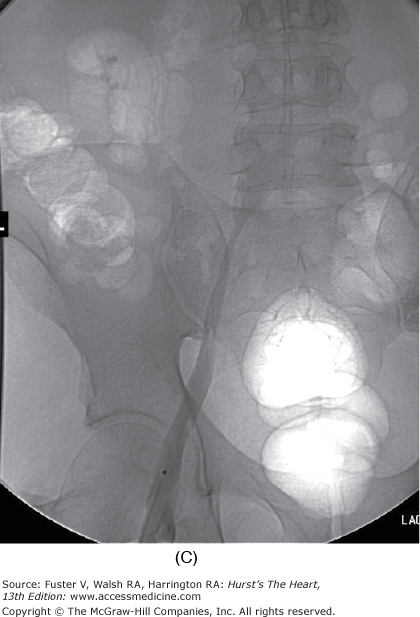
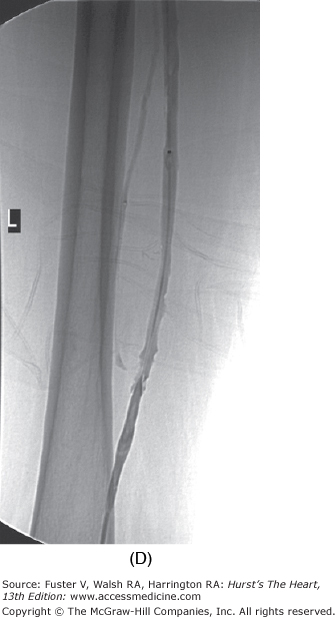
The duration of treatment with warfarin for optimal risk-to-benefit ratio following DVT is not known. Recent literature suggests treatment for a minimum of 6 months in patients with spontaneous DVT and 3 months for a precipitated DVT, but this decision must be individualized for each case.40 The international normalized ratio (INR) should be followed to ensure consistency between laboratories. Patients should be urged to know and record their INR. The risk of major hemorrhage from anticoagulation is 1% to 3% per year when control is strict. Bleeding risk increases significantly when the INR exceeds 4.0.41 Catheter-directed thrombolysis or mechanical thrombectomy for DVT when performed early accelerates recovery and may reduce incidence and severity of postphlebitic syndrome. Lysis appears to be most effective for iliofemoral DVT (Fig. 109–2). However, clearly defined indications and guidelines for thrombolytic therapy in DVT are not yet established.42 Immediate and long-term use of compression stockings to the knee or higher drastically reduces the incidence of postphlebitic syndrome, venous stasis changes, and venous ulceration.43 Strict bed rest is not beneficial or protective in the setting of acute DVT.44-46
The formation of venous ulceration following DVT is unfortunately common and results in great economic impact in developed countries. Ulcers occur at the medial perimalleolar region most often because this area has the highest venous pressure when upright. The excess venous pressure results in a decreased perfusion pressure and chronically hypoxic skin prone to injury.47 Once ulceration has occurred, successful management requires reduction of the edema by compression and conservative debridement of necrotic tissue and fibrinous eschar. After skin integrity is restored, control of venous hypertension with elastic support hose is required indefinitely. When venous ulceration occurs in the setting of moderate to severe PAD, treatment is much more difficult. Compression must be applied with a low-stretch wrap to prevent edema accumulation while avoiding further reduction of arterial inflow.
Phlegmasia cerulea dolens is a rare complication of DVT characterized by acute, massive edema, severe pain, and cyanosis in the setting of extensive iliofemoral thrombosis. One-third of patients die because of pulmonary embolism, and half develop distal gangrene caused by thrombus-induced compartment syndrome. Phlegmasia cerulea dolens is seen most commonly with advanced malignancy or severe infections but can occur following surgery, fractures, and other common precipitants of thrombosis. Treatment includes placement of a caval filter, heparinization, and often physical removal of the clot by thrombectomy (surgical or endovascular) and possibly thrombolysis.48
Peripheral Arterial Disease
PAD caused by atherosclerosis is the most common cause of lower extremity ischemic syndromes in Western societies.49 Symptoms of PAD are variable and, unfortunately, frequently lead to incorrect diagnoses.50 Risk factors for PAD are the same as those for coronary artery disease (CAD), with tobacco and diabetes having an even greater effect51 (Table 109–3). Tobacco use, current and past, is associated with a two- to four-fold increase in relative risk for PAD.52 Diabetes mellitus has a similar increase in relative risk.53,54 Other modifiable risk factors include hyperhomocysteinemia, hyperlipidemia, and hypertension.55
| High Risk | Moderate Risk | Low Risk |
|---|---|---|
| Two- to four-fold increase | One- to three-fold increase | One- to two-fold increase |
| Smoking | Hypertension | Hypercholesterolemia |
| Diabetes mellitus | Homocysteinemia |
PAD affects a large and increasing number of patients worldwide. Exact numbers for prevalence and incidence are confounded by varying methods for assessment and criteria for diagnosis.56 It is estimated that 10 million people have symptomatic PAD and another 20 to 30 million have asymptomatic disease. Ten percent of individuals over age 60 are affected, and prevalence continues to increases with age.57 In the Framingham Offspring Study, prevalence of PAD was determined in 1554 males and 1759 females from 1995 to 1998.58 The mean age was 59 years. PAD, defined as an ankle-brachial blood pressure index (ABI) of <0.90, was present in 3.9% of males and 3.3% of females. Lower extremity bruits were present in 2.4% of males and 2.3% of females. However, the prevalence of claudication was only 1.9% in males and 0.8% in females, suggesting that only half of men and only a quarter of women had symptoms or recognized their symptoms. The PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) study assessed the prevalence of PAD in patients older than age 70 and those age 50 to 69 with a smoking history or diabetes at 250 primary clinics across the United States.59 PAD was defined by a charted or screening ABI of <0.90; 29% of the population was found to have PAD. Nearly half of those with PAD had concurrent coronary or cerebral vascular disease.
Death directly due to PAD is rare. Mortality and morbidity are more often due to concomitant coronary or cerebrovascular disease. The relative risk of death for all-cause mortality is two- to six-fold higher in PAD patients versus the general population.60-62 The risk of death increases as the ABI decreases.63 The 5-year mortality rate for an ABI <0.85 is 10%; when the ABI is <0.40, mortality approaches 50% per year.64 Lower extremity symptoms not associated with a decrease in the ABI do not demonstrate an increase in mortality.65 In contrast, a decrease in ABI without symptoms still portends an increase in cardiovascular morbidity and mortality.66 The Atherosclerosis Risk in Communities study and other studies have demonstrated that an ABI >1.40 is associated with an increase in morbidity such as foot ulcers, congestive heart failure, and stroke but not an increase in cardiovascular events.67,68 Recent reports have indicated that an ABI between 0.90 and 0.99, which is classified as low-normal, increases the risk of future walking impairment.69
Although the cardiovascular mortality and morbidity of patients with PAD is sobering, the rate of progression of limb symptoms and need for limb revascularization or amputation are low.70 Need for revascularization due to tissue loss (ulcer) or rest pain is 5% per year.56 Amputation rates are even lower, at approximately 1% per year.71 The rate of progression and amputation in those who continue to smoke is more than two-fold higher than the rate in those who quit, with 15% of those who continue smoking undergoing amputation within 5 years.72 Historically, diabetics have an amputation rate of 25% over 10 years, which has changed little over time.73,74 For those who present with acute critical limb ischemia, 30-day amputation rates are 10% to 30%, with a 1-year mortality rate of 15%.75
The differential diagnosis of claudication is broad; an abbreviated list is given in Table 109–4. With this in mind, information regarding risk for atherosclerosis, current medical problems, prior lower extremity and back trauma, and all vascular and orthopedic procedures should be obtained. The description of claudication symptoms is unique to each patient.50 Symptom specifics, including onset, progression, and aggravating or alleviating factors, should be rigorously clarified. Commonly, several types of discomfort caused by several etiologies are present.76
| Atherosclerosis obliterans |
| Arteritis (Takayasu, giant cell) |
| Embolic disease/acute arterial occlusion |
| Degenerative joint disease (hip, back, knee) |
| Spinal stenosis |
| Myopathy |
| Thromboangiitis obliterans |
| Popliteal entrapment |
| Venous claudication/varicosities |
| Baker cyst |
| Deconditioning |
| Aortic dissection |
| Aortic coarctation |
| Retroperitoneal fibrosis |
Claudication, literally meaning limping (Latin), is a stereotypical, reproducible distress in single or multiple muscle groups of the lower extremity brought on by sustained exercise and relieved by rest. The distress is classically described as cramping, but numbness, weakness, giving way, aching, and dull pain are all common adjectives.77 The distress changes in character and/or location as the flow-limiting lesion(s) progress. When workload is increased by rapid pace, a burden, or walking uphill or over rough terrain, the distance or time to onset will shorten. When the distance to onset or severity abruptly changes, thrombosis in situ or an embolic event should be considered. In general, symptoms occur distal to the level of stenosis or occlusion. Claudication occurs in muscle groups rather than joints. Relief with rest is independent of position and is timely, usually complete within 5 minutes. When specific positions are required for relief, musculoskeletal or neurologic disorders should be suspected. Claudication often worsens after a period of inactivity, such as hospitalization for another reason, but returns to baseline with reconditioning. Although lifestyle limitation and changes in quality of life are an integral part of the history, quantification of disease severity by history alone is unreliable.34 Standardized treadmill testing using ABIs at rest and after completion of an exercise protocol confirms the diagnosis, determines the severity, and documents claudication distance for future follow-up.77-79
Critical limb ischemia may be defined as tissue loss or rest pain in a limb, usually at the distal portion and rarely in the entire limb. Small, localized areas of ischemic pain or ulceration are often caused by minor trauma to an area with poor perfusion rather than progression of disease.77 It is important to inquire about new shoes, recent nail care, pets, and other potential sources of trauma. Rest pain is present when supine and is often relieved by dependency, such as hanging the limb off the bed or, paradoxically, by walking. Pain may progress to become constant, interrupting sleep, suppressing appetite, inducing weight loss and delirium, and requiring large doses of analgesics for pain relief. Patients may take to sleeping in a chair to get better rest and often will present with edema as a result.
Pseudoclaudication is typically of neurogenic origin. The patient with neurogenic claudication describes exercise-induced distress with a dysesthetic quality that clears slowly or requires a specific posture for relief, usually with the hips flexed.80 Clumsiness may develop as walking continues. Symptoms also occur with prolonged standing or when supine. Compression of the distal spinal cord by hypertrophic bone, disk protrusion, or tumor may be the cause. A history of back injury is common. Arterial and pseudoclaudication often coexist. In this situation, the dominant lesion can be clarified by observing and timing symptoms with standing still and comparing with symptoms during exercise, as well as by measuring the arterial indices before and after exercise.81
Venous claudication is described as a congestive, often bursting, distress of thighs and calves induced by standing, walking, and sometimes running. Relief with rest is slow and notably accelerated when the patient elevates the legs. Venous claudication occurs in the setting of iliocaval obstruction. Signs of venous hypertension of the legs and lower abdomen are often noted during examination.82
A red or purplish color of the forefoot during dependency (dependent rubor) is common with severe ischemia. Rubor caused by ischemia will change to pallor with elevation, versus cellulitis that may remain rubrous with elevation.83 Timing the onset of pallor and time to venous refilling can be performed in the examination room (Table 109–5). Loss of normal hair growth is also a marker of ischemia.
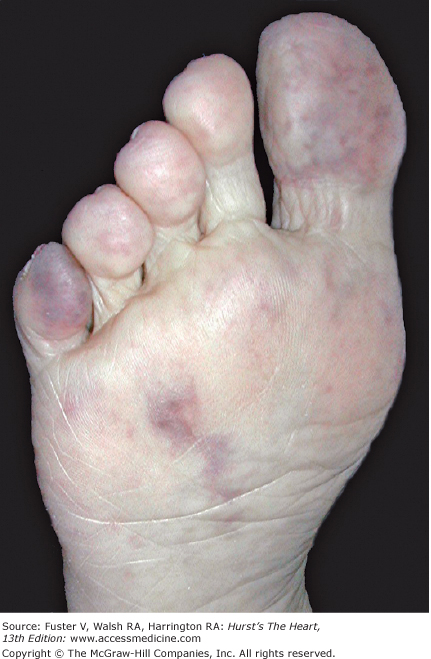
Livedo reticularis is a transient, bluish discoloration with a lacy pattern found on the extremities and sometimes the trunk that is variable in its extent and intensity. It is most apparent after exposure to cold or emotion and fades with warming or exercise. It is more common in women and fair-skinned individuals. Livedo reticularis is common, and when mild, it is often overlooked. It is postulated that spasm of the cutaneous arterioles (with secondary dilation of the capillaries and venules) causes slow flow, increased oxygen uptake, and reduced oxygenation of hemoglobin, producing the color change. Secondary livedo reticularis is patchy, focal, and asymmetric in distribution and typically fixed. It may be complicated by local infarction or ulceration (Fig. 109–3). Embolism is a common etiology following instrumentation. The lesions may be elevated or tender when caused by vasculitis.
The aorta, radial, ulnar, subclavian, carotid, temporal, occipital, femoral, popliteal, posterior tibial, and dorsalis pedis arteries are accessible by palpation. Pulses are graded on a scale (Table 109–6).84 If a pulse is not palpable, Doppler examination should be performed to establish whether flow is absent or below the level of detection by palpation. Surface temperature is reduced when perfusion is compromised. Temperature differences are best felt with the dorsum of the fingers; comparison to the contralateral limb or proximal ipsilateral limb should be made. The sizes of paired arteries are similar in magnitude. Ectasia or aneurysm is suspected when one side is larger or more forceful than the other. Tortuosity of the carotids, abdominal aorta, and subclavian arteries can mimic an aneurysm. Ultrasound or other imaging studies are needed to clarify the findings when the diagnosis of an aneurysm is entertained.
| Mayo Grade | Physical Findings | ACC/AHA Grade |
|---|---|---|
| 0 | Absent | 0 |
| 1 | Severely reduced—palpable with great difficulty; unable to accurately count pulse | 1 |
| 2 | Moderately reduced—palpable with some difficulty; able to count pulse | 1 |
| 3 | Mildly reduced—easily palpable | 1 |
| 4 | Normal pulse—easily palpable | 2 |
| 5 | Enlarged—widened, possibly aneurysmal | 3 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree