Chapter 90 Devices for the Management of Atrial Fibrillation
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice and occurs most often in association with cardiovascular disease. AF incidence increases with age and affects 2% of the population older than 65 years and 4% of the population older than 80 years, with a median age of the AF population being approximately 75 years.1,2 Patients with AF have a near doubling of cardiovascular mortality rate in men and a 50% increase in women.3 In addition to symptoms of palpitations, patients with AF have an increased risk of stroke and may also develop decreased exercise tolerance and left ventricular dysfunction. AF is a frequent and costly health care problem representing the most common arrhythmia, accounting for more than one third of all admissions for arrhythmias.
The importance of antithrombotic therapy is now undisputed, but the management of the arrhythmia itself remains controversial. Although prospective studies as Rate Control Versus Electrical Cardioversion for Persistent Atrial Fibrillation (RACE),4 Strategies of Treatment of Atrial Fibrillation (STAF),5 and Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM)6 have not shown improved morbidity or mortality rates with rhythm control, effective rhythm control was not often achieved in these studies due to the use of an antiarrhythmic drug–based strategy. The inability to maintain rhythm control is due to the limited efficacy of antiarrhythmic drugs, a finding that has been repeatedly documented in clinical trials. In the STAF study, only 23% of the patients actually achieved freedom from AF on amiodarone therapy.5
Pathophysiologic Basis for Device Therapy in Atrial Fibrillation
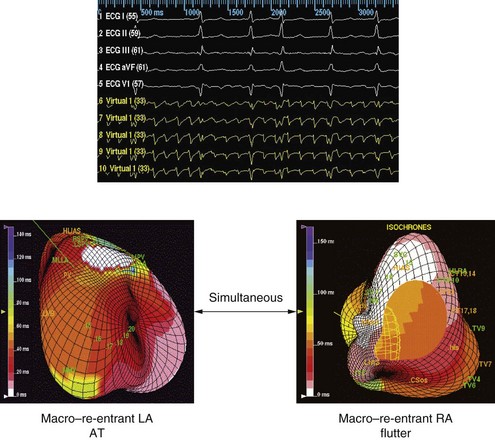
Significant new knowledge has emerged regarding potential mechanisms of AF in different patient populations as well as the impact of therapies on these mechanisms. AF in humans depends on the interaction between triggers and a vulnerable substrate for facilitating the process of functional, anatomic, or spiral wave re-entry. These triggers consist of automatic or autonomically triggered atrial premature depolarizations (APDs) or organized automatic or re-entrant atrial tachycardia (AT). Triggers may emanate from the right atrium (RA) or left atrium (LA), particularly in the presence of structural heart disease.7 Enhanced sympathetic tone increases the frequency of triggers, and conditions of vagotonic bradycardia can shorten atrial effective refractory periods and increase dispersion of atrial refractoriness. Perpetuation of AF is associated with structural and electrical remodeling of the substrate.8 These changes include areas of intra-atrial or interatrial conduction delay, shortening and dispersion of the atrial refractoriness, and a loss of rate adaptation. Atrial fibrosis is common, increases with age, and progresses to heart failure. This substrate provides the milieu for conduction delay, re-entry, and/or vortex shedding of daughter wavelets. Consequently, organized monomorphic AT (Figure 90-1) have been demonstrated during the onset and maintenance of AF.9,10 Further knowledge has emerged regarding the determinants of endocardial septal activation and conduction over interatrial connections. It has been shown that electrical coupling of the right and LA in humans is predominantly provided by muscular connections at the level of Bachmann’s bundle and the coronary sinus. The true septum (the fossa ovalis and its limbus) of the RA and LA is asynchronous and discordant, usually without contralateral conduction during sinus rhythm or atrial pacing.11
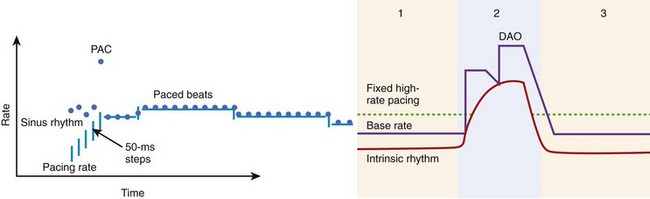
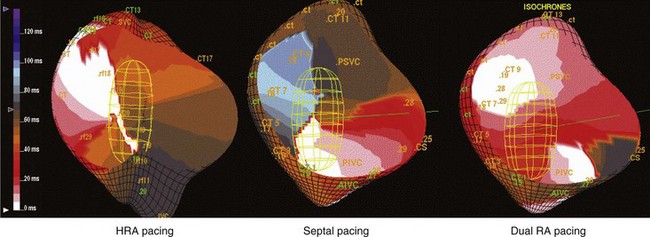
The rationale for pacing therapy can now be refined on the basis of this new knowledge. Atrial-based pacing can prevent AF by several mechanisms. Thus its effect on triggers, initiating or onset arrhythmias, mechanisms that maintain AF, and their contributions can be examined in different patient populations. Trigger density is a critical element in the high event rate for asymptomatic and symptomatic AF events seen in patients with refractory AF. Although antiarrhythmic drugs have been commonly used to suppress atrial ectopic beats, overdrive atrial pacing and novel atrial pacing algorithms can reduce ectopy (Figure 90-2). Overdrive pacing can also suppress triggers, such as automatic AT or atrial premature depolarizations.12,13 Alternate-site pacing can reduce atrial conduction delay and shorten the activation time. Atrial-based pacing can also affect the electrophysiological properties of the substrate by pre-exciting regions critical to re-entry, such as sites of conduction delay.14,15 Reduced interatrial and intra-atrial conduction delay and dispersion of refractoriness have been shown with septal and multi-site atrial pacing (Figure 90-3). With dual-site right atrial or biatrial pacing, regional atrial activation is resynchronized. Hesselson and Wharton demonstrated acute suppression of pulmonary vein foci with overdrive right atrial pacing, but suppression of AF occurred only when dual-site atrial pacing was used.15 Dual-site right atrial pacing and Bachmann’s bundle pacing reduce or markedly truncate the window of AF induction by APDs.14,16 Dual-site right atrial pacing has also been shown to improve atrial mechanics by increasing atrial filling velocities and preventing the left ventricle (LV) and LA dilation seen with right ventricle (RV) apical pacing (see Figure 90-3).17 Furthermore, dual-site right atrial pacing has been shown to increase left atrial appendage flow and to shift the transmitral flow pattern toward a lower passive component in patients with sick sinus syndrome and paroxysmal AF.18 Multi-site pacing resulted in in earlier left atrial contraction and reversal of the typical right-left atrial contraction sequence as well as providing greater left atrial contraction synchrony.19,20
Recent data from the Atrial Fibrillation Therapy (AFT) trial show that sinus bradycardia preceded onset of AF in 22% of study patients.21 This deviation in sinus rate can be modest, greater than 5 beats/min or more and transient (5 minutes or more) but can create the opportunity for triggering AF. Demand atrial pacing can prevent this event. Finally, common right atrial isthmus-dependent atrial flutter is an important and hitherto underrecognized trigger for AF. Treatment of this rhythm with anti-tachycardia pacing (ATP) can be effective in its termination and AF prevention.
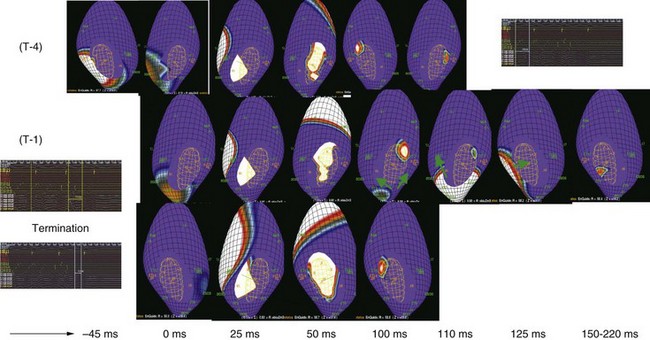
The second part of the arrhythmogenic chain is the onset or transitional tachycardia in AF. This tachycardia can be transient or prolonged in duration and can simulate AF on the electrocardiogram (ECG). Mapping has demonstrated such rhythms in both spontaneous and induced AF.7,22 Three-dimensional mapping of individual atria has characterized these rhythms as macro–re-entrant AT (see Figure 90-1), common atrial flutter, and atypical atrial flutter.22,23 Multi-site pacing can reduce or eliminate the initiation of these arrhythmias by altering substrate properties (Fig. 90-4). ATP can be used for termination of these rhythms and prevent progression to sustained AF (Figure 90-5).24
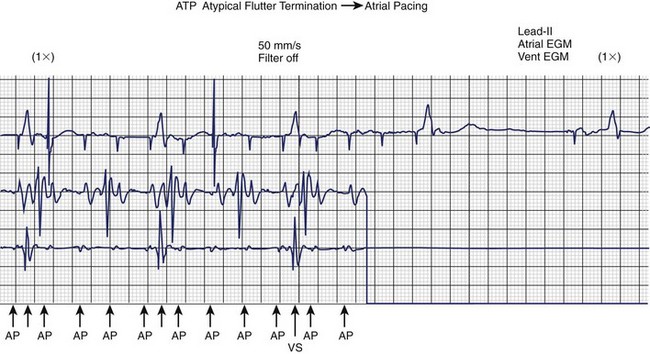
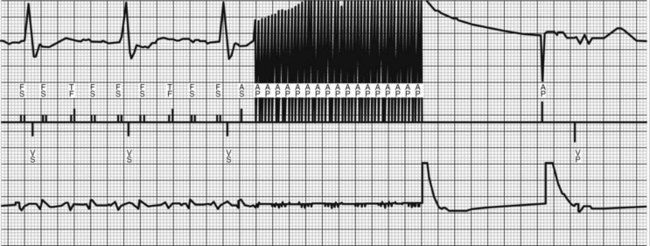
FIGURE 90-5 Rapid atrial anti-tachycardia pacing (ATP) terminates atypical atrial flutter in this patient with permanent atrial fibrillation (see Figure 90-1) implanted with a Guidant pacemaker (Boston Scientific, Inc.) and later with a Guidant Vitality AVT dual defibrillator with dual-site atrial pacing. The patient reverts to an atrial paced (AP) rhythm. EGM, Electrogram; VS, ventricular sensed event.
Evolution to sustained AF requires electrophysiological mechanisms that result in perpetuation of the arrhythmia. More than one re-entrant tachycardia may coexist in this phase, or a single tachycardia may persist; both models can demonstrate fibrillatory conduction.25 Critical requirements for such persistence include structural and/or electrophysiological remodeling of the AF substrate. Atrial conduction delays manifest as prolonged or abnormal P waves or prolonged atrial conduction intervals and are a prime predictor of AF recurrence. Resynchronization therapy with multi-site atrial pacing can reduce this delay in multiple atrial regions. Dual-site right atrial pacing therapy may have novel effects that lead to reverse remodeling of the atrial substrate by improving atrial transport and reducing atrial stretch. Recently, Avitall and coworkers demonstrated a significant benefit in the prevention of AF by simultaneous high-density biatrial pacing directly targeted to the pulmonary vein antra and other LA locations known to be AF triggers, compared with single-site pacing. By using a multi-site simultaneous pacing protocol, they showed favorable changes in one of the key electrophysiological parameters that contribute to AF, slowed atrial conduction, specifically in response to single premature atrial contractions.26
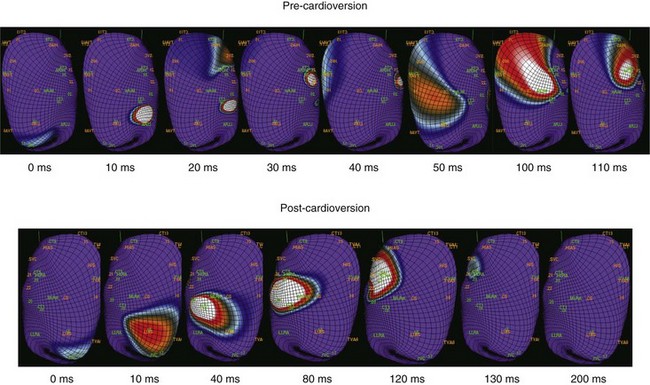
Termination therapies, such as ATP, can terminate common and non-isthmus-dependent atrial flutter by burst, ramp, or a combination of rapid pacing sequences. This can interrupt the chain of AF development or its maintenance. In our laboratory, 50-Hz pacing (Figure 90-6) has been shown to terminate atypical non-isthmus-dependent atrial flutter but not sustained AF.27 Atrial defibrillation shocks produce conduction delay and block (Figure 90-7).
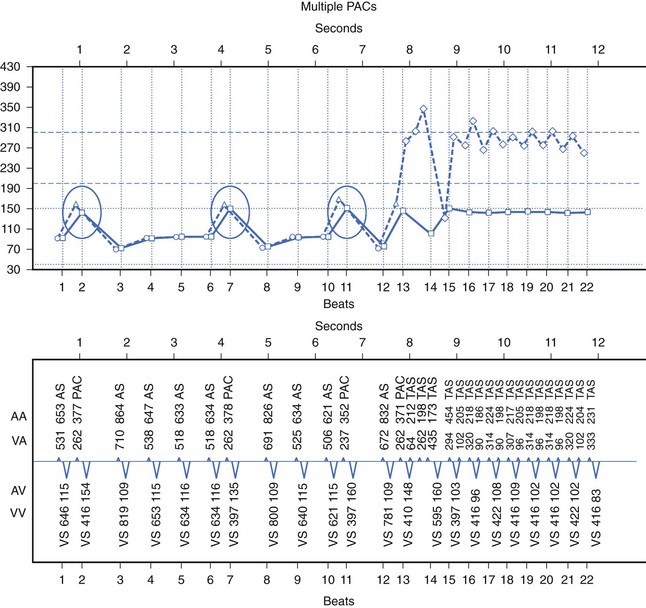
Although extensive mapping data are unavailable for internal atrial defibrillation, mapping of external shocks demonstrates that even ineffective shocks modify the underlying atrial activation patterns in AF, resulting in transient or persistent slowing.28 Successive shocks may be effective when the first one modifies the sustained AF mechanisms, resulting in vulnerability to the next shock or even spontaneous termination mediated by an unstable circuit or concealed penetration by an atrial premature beat (Figure 90-8).
Initial studies on catheter atrial defibrillation used two small surface area defibrillation electrodes and monophasic shocks. Defibrillation could be accomplished with shock energies of less than 1 J but was poorly reproducible.29 Ventricular fibrillation was occasionally induced (2.4%) when shocks were delivered more than 115 ms after QRS onset. Kumagai and coworkers reported efficacy rates of 47% at energies less than 0.5% and 74% at 1 J.30 Biphasic shocks are associated with lower thresholds than are monophasic shocks.31 Experimental studies have demonstrated that the right atrium to coronary sinus defibrillation vector is associated with the lowest defibrillation thresholds.32 This optimized waveform with individual phase durations of 3 ms and the optimal shock vector (E50) has been estimated at 1.3 J. In the sheep model, bi-phasic shocks with an RA-left thoracic patch vector had a 47% efficacy at 1 J.33 Epicardial atrial defibrillation thresholds have been studied and have shown extremely low energy needs with an efficacy of 42% in the 0.3 J range.34 In contrast to clinical data, these experimental studies suggested that atrial defibrillation could be achieved at values below the pain perception threshold for shock therapy.
Atrial Pacing for the Primary Prevention of Atrial Fibrillation
Pacing to Prevent Atrial Fibrillation After Cardiac Surgery
Postoperative AF is a frequent complication, occurring in 30% to 50% of patients after cardiac surgery.35 The utility of prophylactic atrial pacing to prevent AF after cardiac surgery has been investigated in a number of trials, but clinical guidelines for its use are lacking.36,37 Nine randomized, controlled trials addressed prophylactic atrial pacing after cardiac surgery to prevent AF.38 Prophylactic right atrial pacing and prophylactic left atrial pacing have yielded inconclusive results. Prophylactic bi-atrial pacing reduced the incidence of AF significantly in four studies,39,40 reduced it nonsignificantly in one study, and had no effect in one study.41,42 Newer pacing techniques such as high-frequency stimulation of the right inferior fat pad have yielded a significant decrease in ventricular rate during postoperative AF.43 On the basis of the literature, it can be concluded that prophylactic atrial pacing to prevent AF after cardiac surgery is safe. We recommend that bi-atrial pacing be considered, particularly in patients who are at high risk for the development of postoperative AF. Several mechanisms have been advocated to explain the high incidence of postoperative AF.44 Besides mechanical effects, systemic inflammatory responses have been considered of greatest importance. Analysis of several electrocardiographic parameters has shown that premature atrial contraction activity, frequency-domain heart rate variability, and heart rate turbulence parameters were the best discriminators for postoperative AF occurrence.45 These data suggest that dual-site pacing may be effective in primary prevention in certain populations and could enhance efficacy. The Pacing of the Atria in Sinus Node Disease (PASTA) trial has been designed to re-examine this issue by comparing the impact of different atrial pacing lead positions on the incidence of AF in patients with sinus node disease. The evaluation of an AF burden greater than 1% and the total AF burden after 24 months did not show differences in the incidence of AF in patients with dual-chamber pacemaker therapy for sinus node disease. In addition, a significant influence of RA lead position on the incidence of AF recurrence could not be demonstrated.46
Pacing Mode and Atrial Fibrillation
The role of permanent pacing to prevent AF has been highlighted in several reviews.47–51 Many retrospective and several prospective, randomized, controlled clinical trials have reported that atrial or dual-chamber pacing prevents paroxysmal and permanent AF in patients with symptomatic bradycardia as an indication for cardiac pacing.52–54 The Danish Trial of Physiologic Pacing (DTPP) in sick sinus syndrome was the first prospective trial comparing atrial-based pacing in the AAI mode with an active control arm using ventricular demand (i.e., VVI pacing). Despite initial equivalence, long-term follow-up (mean, 5.5 years) demonstrated a 46% relative risk reduction in the incidence of AF with atrial pacing.52 These findings suggest a slow reverse atrial remodeling effect with atrial pacing. The Pacemaker Selection in the Elderly (PASE) study53 was conducted to assess specifically the effect of pacing on health-related quality of life. No difference in the development of AF was observed in the PASE study between the two pacing modes, but those with sinus node dysfunction as the primary indication for pacing who were randomized to the dual-chamber pacing mode tended to be less likely to have AF (19%) compared with those randomized to the ventricular pacing mode (28%, P = .06).53
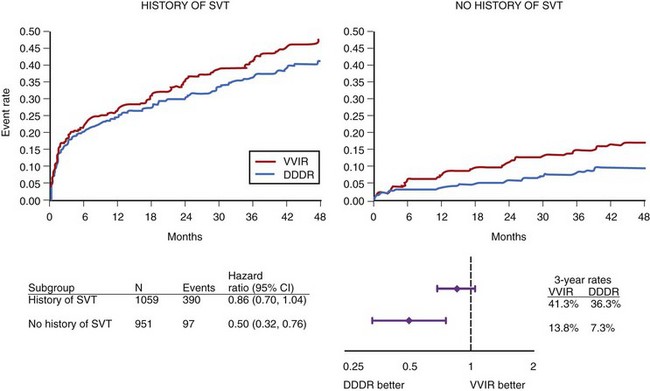
The three largest parallel-design trials randomizing patients to VVI(R) or DDD(R) pacing, the Canadian Trial of Physiologic Pacing (CTOPP),54 the Mode Selection Trial (MOST),55 and the United Kingdom Pacing and Cardiovascular Events (UKPACE)56 trial have demonstrated no mortality rate benefit from physiological mode selection. CTOPP54 reported an 18% risk reduction in the development of AF over 3.1 years (P = .05) in patients randomized to physiological pacing compared with ventricular pacing, which was sustained over longer term follow-up (20% risk reduction; P = .009 at 6 years).57 MOST55 showed a 21% relative risk reduction for developing AF over 3 years in patients randomized to physiological pacing compared with ventricular pacing (P = .008) (Figure 90-9). It is worth noting that during the trial, 31% of the ventricular pacing group crossed over to physiological pacing, potentially diluting any benefit relating to this therapy. A similarly high crossover rate (26%) to dual-chamber pacing among patients assigned to ventricular pacing was observed in PASE.53 UKPACE randomized 2021 patients aged 70 years or older with high-grade AV block to implantation with dual-chamber or single-chamber ventricular pacemakers, and further subdivided the single-chamber population into two groups: fixed-rate ventricular pacing (n = 504), and rate-modulated single-chamber pacing (n = 505).56 After a median follow-up period of 4.6 years for the primary endpoint, all-cause mortality, and 3 years for cardiovascular events and AF (2.8% DDD vs 3.0% VVI; P = .74), no significant differences were found between physiological and ventricular-based pacing.58
A meta-analysis of these trials,59 which included 35,000 patient-years of follow-up, showed that atrial pacing is superior to ventricular pacing and resulted in a significant reduction in AF (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.72 to 0.89; P =.00003) and conferred a modest reduction in stroke risk (HR, 0.81; P = .038). Furthermore, subgroup analysis suggested a greater benefit of atrial pacing in patients with sinus node dysfunction in terms of stroke and cardiovascular death, but these results must be interpreted cautiously. An important consideration that may have limited the benefit of atrial pacing in the prevention of AF is the observation that most patients in these trials received DDD pacemakers, and the resultant increase in right ventricular pacing may have counterbalanced the reduction in the incidence of AF. Mode selection, however, did not affect all-cause mortality and stroke or heart failure hospitalization in the overall group.59
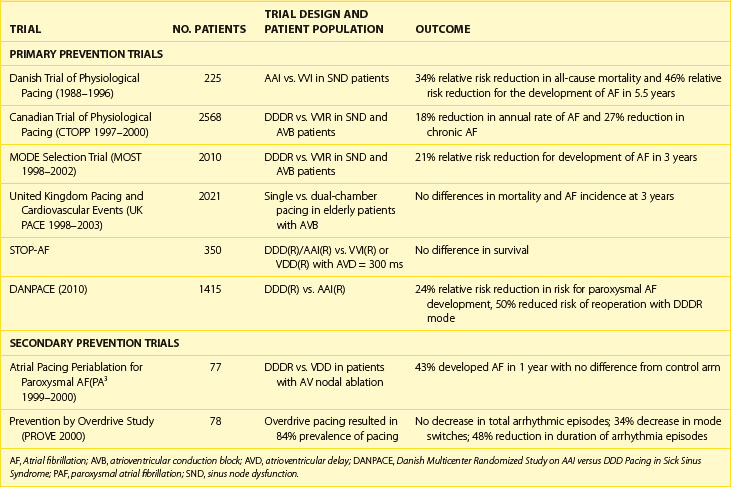
Primary prevention trials of atrial-based pacing are summarized in Table 90-1. They uniformly demonstrate a relative risk reduction in the propensity to develop AF in patients with sinus node dysfunction during long-term follow-up. Interestingly, a greater risk reduction was seen with single-site atrial pacing than with dual-chamber pacing modes, with the exception of the Danish Multicenter Randomized Study on AAI Versus DDD Pacing in Sick Sinus Syndrome (DANPACE). In this study, AAIR and DDDR did not differ with respect to survival, stroke risk, or development of chronic AF. Paroxysmal AF was reported more frequently with the AAIR mode, but surveillance did not include full-disclosure device data logs. In contrast, patients with AV block do not show this reduction in AF incidence with dual-chamber pacing. This could potentially be due to less use of atrial pacing in this population or disease localized to the AV junction. Perhaps the higher levels of ventricular pacing inherent with DDD devices and conventional programming, including ventricular dyssynchrony, were negating the potential benefits of maintaining AV synchrony, explaining the modest benefits observed in the individual dual-chamber pacing trials.
Atrial Pacing for the Secondary Prevention of Atrial Fibrillation
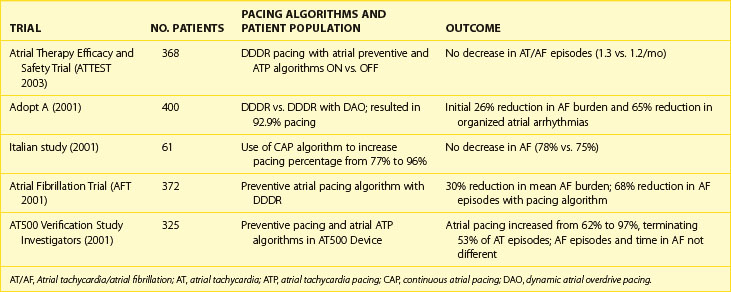
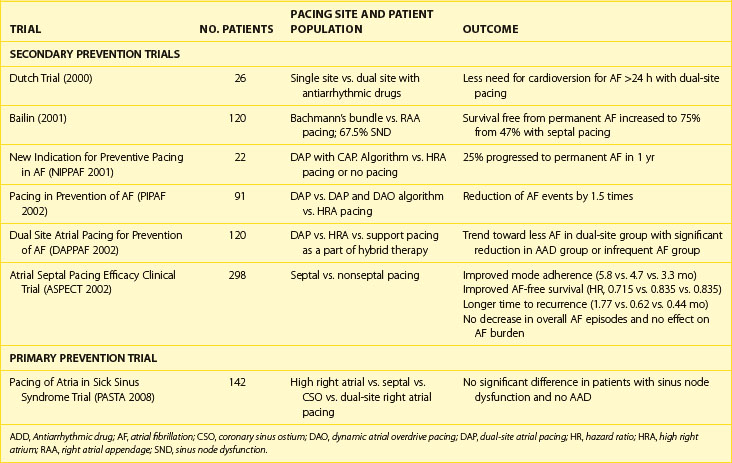
Clinical investigation of atrial pacing techniques for management of AF in symptomatic AF populations has been performed in several high-profile prospective clinical trials.60–65 Analysis of the clinical benefit of atrial pacing is complicated by limited knowledge of the natural history of AF in different AF populations and by a lack of standardized endpoints for quantifying clinical benefit. Many studies lack a control group without atrial pacing therapy to judge efficacy. Approaches to date have included standard high right atrial or dual-chamber pacing alone or with rate response (see Table 90-1), right atrial pacing with novel pacing algorithms (Table 90-2), or alternate-site (high or low septal pacing) and dual-site atrial pacing (Table 90-3) and newer pacing algorithms minimizing ventricular pacing.
Site-Specific Atrial Pacing for Prevention of Atrial Fibrillation
Standard Right Atrial Pacing
The efficacy of standard high right atrial pacing alone for prevention of symptomatic paroxysmal AF has been evaluated in clinical studies and currently remains unproven. High right atrial pacing alone for prevention of symptomatic drug-refractory paroxysmal AF patients without bradycardia who are awaiting AV junctional ablation was evaluated in a prospective, randomized, crossover study by Gillis and coworkers, the Atrial Pacing Peri-Ablation for the Prevention of AF (PA3) trial.60 They observed no prolongation in the time to recurrent AF compared with placebo. In patients with refractory AF as the sole arrhythmia, only the Jewel AF device (Medtronic, Inc., Minneapolis, MN) trial showed that high right atrial pacing appeared to reduce frequency but not AF burden initially, but more detailed analysis failed to confirm long-term benefit.61 The Prevention by Overdrive study (PROVE), a randomized crossover trial, evaluated a similar device, the Talent DR 213 pacemaker (ELA Medical, Paris, France), combining atrial overdrive pacing with an automatic rest rate function.62 Overdrive pacing resulted in an improved atrial pacing percentage (84%) but did not reduce the total number of episodes, although there appeared to be a reduction in AF episode duration.
Another approach using high right atrial pacing in combination with other antiarrhythmic therapies, such as drugs, can also be considered but has not been formally studied in prospective studies. In an early experience from our group, antiarrhythmic drug therapy combined with high right atrial or septal pacing prolonged arrhythmia-free intervals, but no long-term data were available on rhythm control.63
Alternate-Site Atrial Pacing
Alternative single-site pacing involves placement of a septal RA lead. This can be further subdivided into high (Bachmann’s bundle), mid (fossa ovalis), or low septal pacing (triangle of Koch). Alternate-site and multi-site atrial pacing methods, such as dual-site right atrial pacing and bi-atrial synchronous pacing, have been evaluated for AF and atrial flutter prevention (see Table 90-3). Several studies of atrial septal pacing have shown a decrease in AF frequency and burden compared with right atrial appendage pacing, whereas other studies have not observed any difference in short-term follow-up.64–67 Bailin and colleagues64 randomized 120 patients with paroxysmal AF and bradycardias to high septal pacing or right atrial appendage pacing. Patients with high septal pacing had improved freedom from permanent AF at 1 year compared with standard right atrial pacing (75% vs. 47%), but there was no observed decrease in symptomatic AF event frequency. Despite improvement, a significant proportion (25%) of patients progressed to permanent AF. A control arm without pacing therapy was absent in this trial. With a similar study design, Hermida et al reported their findings in patients without AF as an inclusion criterion.65
The Atrial Septal Pacing Efficacy Clinical Trial (ASPECT)66 randomized patients to low septal or standard right atrial pacing and used preventive pacing algorithms with the Medtronic AT500 pacemaker. In this study, low septal pacing was not associated with a reduction in AF frequency or burden. De Voogt et al performed the Overdrive Atrial Septum Stimulation (OASES) study67 and examined the effect of atrial overdrive in patients with right atrial appendage and low interatrial septal pacing in 177 patients. With a 3-month crossover design, they found no significant effect of atrial overdrive on AF burden or mode switch number in either the right atrial appendage or septal paced groups.
Compared with multi-site atrial pacing, these alternative pacing sites require less hardware. The relative efficacy of right atrium high versus low atrial septal pacing for AF prevention remains unknown; however, the use of high septal pacing is associated with a lower risk of ventricular far-field sensing. Despite these findings, it is important to note that no large multicenter clinical trial of alternative-site pacing to prevent AF has been performed. Until these types of data are available, the use of alternative-site pacing should be considered unproven.49
Multi-Site Atrial Pacing
Biatrial Pacing
Synchronous bi-atrial pacing appears to be an interesting therapeutic modality for the management of refractory atrial arrhythmias to correct the deleterious electrophysiological consequences of high-grade intra-atrial or interatrial conduction disorders. In the majority of patients, the device implanted featured three pacing leads: two atrial leads linked by a bifurcated Y-connector to the atrial port of a dual-chamber pacemaker, the RA lead in cathodic configuration and the left atrial lead in anodic configuration, and a third lead placed in the right ventricular apex and linked to the ventricular port of the pacemaker. This configuration allowed standard DDD pacing with simultaneous bi-atrial pacing, forming a “triple chamber” pacemaker. The pacemakers were programmed in either triggered mode AAT(R) or DDD(R) mode. Synchronous Biatrial Pacing for Atrial Arrhythmia Prevention (SYNBIAPACE), a randomized crossover study, compared synchronous bi-atrial, high right atrial, and demand pacing during 3-month treatment arms. Patients were included if they had a standard indication for pacing, two or more episodes of AF in 3 months, and a P-wave duration of at least 120 ms. There was a nonsignificant increase in AF-free interval with bi-atrial pacing alone compared with high right atrial pacing.68 Long-term effects of biatrial synchronous pacing in patients with intra-atrial conduction delay and recurrent atrial flutter and fibrillation to prevent recurrent AT/AF have been encouraging but variable.69 In this 9-year follow-up study, at a mean follow-up of 33 months, 64% of the patients remained in sinus rhythm, whereas the others had repeated episodes of paroxysmal AF or developed persistent AF. A majority of these patients were receiving antiarrhythmic drug therapy.
Dual-Site Right Atrial Pacing
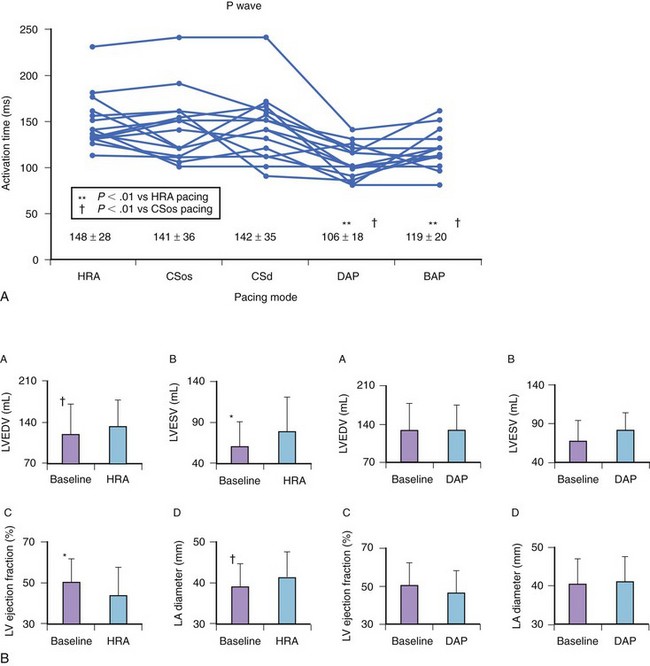
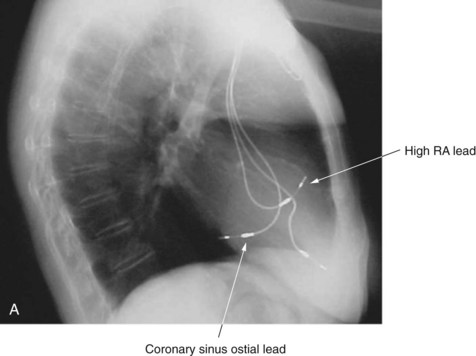
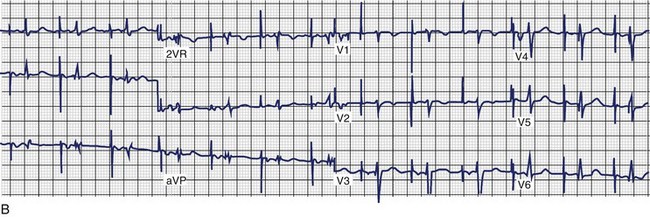
For dual-site right atrial pacing, an additional atrial pacing lead is inserted just outside the coronary sinus ostium for stability and left atrial stimulation (Figure 90-10, A). During simultaneous pacing of the high right atrium and coronary sinus ostium, the ECG shows a biphasic P wave in the inferior leads with abbreviation of P-wave duration (Figure 90-10, B). Several small, randomized trials were initially reported in addition to single-center pilot experiences. In our pilot experience, patients with drug-refractory symptomatic AF and bradycardias showed trends suggesting benefit with dual-site right atrial pacing in 3-month crossover interim analyses, but significant benefit of dual-site over high right atrial or septal pacing was obvious only after 1 year.63 In a short-term randomized, 12-week comparative study of symptomatic AF patients without bradycardia, New Indication for Preventive Pacing in AF (NIPPAF), Lau and coworkers reported an increase in mean time to first AF recurrence from 15 to 50 days in patients during dual-site pacing compared with no pacing.70 These single-center experiences have been followed by short-term randomized, multicenter studies.
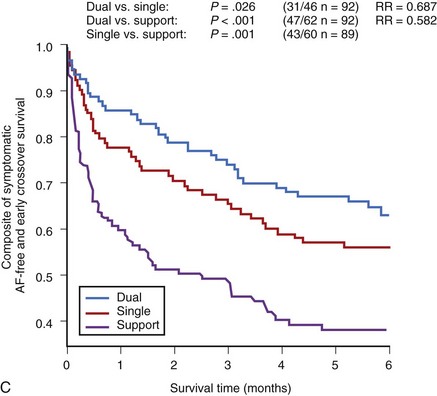
The Dual Site Atrial Pacing for Prevention of Atrial Fibrillation Trial (DAPPAF) was a medium-term multicenter crossover study with 6-month treatment arms comparing dual-site right atrial, high right atrial, and support pacing.71 It enrolled patients with frequent, symptomatic, and drug-refractory AF with bradyarrhythmias requiring cardiac pacemaker insertion. After dual-site right atrial pacing system implant, optimization of drug and pacing therapies was performed. The three modes of pacing were then randomly selected for 6-month periods. Patient tolerance and adherence to the pacing mode were superior in dual right atrial pacing compared with support (P < .001) and high right atrial pacing (P = .006) (Figure 90-10, C). Freedom from any symptomatic AF recurrence tended to be greater with dual right atrial (HR, 0.72; P = .07) but not with high right atrial pacing (P = .19) compared with support pacing. Combined symptomatic and asymptomatic AF frequency in patients was significantly reduced during dual right atrial pacing compared with high right atrial pacing (P < .01). However, in antiarrhythmic drug–treated patients, dual right atrial pacing increased symptomatic AF-free survival compared with support pacing (P = .011) and high right atrial pacing (HR, 0.67; P = .06). In drug-treated patients with less than one AF event per week, dual right atrial pacing significantly improved AF suppression compared with support pacing (HR, 0.46; P = .004) and high right atrial pacing (HR, 0.62; P = .006). Lead dislodgment was uncommon (1.7%), with coronary sinus and high right atrial lead stability being comparable. Thus DAPPAF showed improved adherence to pacing and rhythm control in the dual-site mode, especially when combined with antiarrhythmic drugs. This study supports the use of a hybrid or “add-on” therapy use of dual atrial pacing for rhythm management. In another prospective crossover trial with 6-month treatment periods performed in patients with recurrent symptomatic AF without structural heart disease, Ramdat Misier and coworkers have shown a significant increase in time to recurrent AF and interventions for symptomatic AF recurrence.72
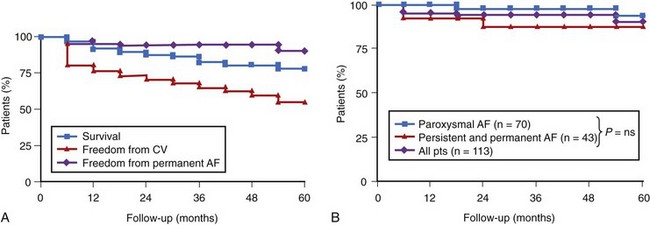
Longer term experience in secondary prevention of drug-refractory AF with dual-site right atrial pacing is now available (Figure 90-11). This experience has been acquired in patients with recurrent, symptomatic, and frequent drug-refractory AF, with or without concomitant bradycardias. In our long-term experience initially involving more than 125 patients with follow-up ranging to 10 years, the overall patient survival rate is 80% at 5 years. Rhythm control, as documented by device data logs, was achieved in more than 90% of patients at 3 years or more of follow-up. The overall stroke incidence was 1.0% per year.73 Similar efficacy rates can be achieved in paroxysmal, persistent, and permanent AF (see Figure 90-11, B). However, occasional cardioversion and linear right atrial ablation was needed in approximately half of the patients with persistent and permanent AF for rhythm control. A nonrandomized, parallel-cohort experience from Europe in patients with bradycardias who required pacing and had paroxysmal AF supports long-term efficacy with dual-site right atrial pacing.74 Of 83 patients, 30 had dual-site right atrial pacing systems and 53 had single-site high right pacemakers implanted. Patients with dual-site systems had a longer duration of AF (8.1 vs. 3.8 years for high right atrial systems; P < .001) and more did not show a positive response to drug trials (2.4 vs. 1.6 for high right atrial systems; P < .05). During a mean follow-up of 18 months, symptomatic paroxysmal AF recurred in nine patients after dual right atrial pacing compared with 24 patients after high right atrial pacing (P = .03). Permanent AF supervened in only one patient after dual right atrial pacing and in 12 patients after high right atrial pacing (P < .05).
Novel Pacing Algorithms
A number of dedicated pacing algorithms have been developed for AF. The potential mechanism(s) of these algorithms are summarized in Table 90-2. These algorithms have been added to the armamentarium of device-based approaches for preventing AF and have been shown to increase the frequency of atrial pacing compared with conventional rate-response pacing. Prevention algorithms can be divided into two groups; continuous or triggered.
Single Algorithm Trials
Atrial Fibrillation in Bradycardia Tachycardia Syndrome
Trials using the novel algorithms for AF suppression in patients with concomitant bradycardia are summarized in Table 90-2. The AT500 pacemaker was a DDDRP device with two preventive atrial pacing leads. Atrial preference pacing changed the base pacing rate in response to atrial premature beats, with a programmable increment. Atrial rate stabilization intercedes after premature beats, altering the post-ectopic escape interval by markedly reducing it and then slowly easing down to the base pacing rate. ATP, as illustrated in Figure 90-5, can terminate common and non-isthmus-dependent atrial flutter by burst, ramp, or combination rapid pacing sequences. These algorithms are now present in the Medtronic EnRhythm pacemaker.
The Atrial Overdrive Pacing (AOP) trial prospectively evaluated overdrive single-site pacing in the right atrium for AF prevention in 145 patients in a randomized study design. This study did not reduce the frequency of AF events as indirectly as assessed by mode switch episodes. No symptomatic improvement was noted, but the pacing mode was well tolerated.75 The Stimolazione Atrial Dinamica Multisito (STADIM) study from Italy compared the efficacy of single- and dual-site atrial pacing, with or without a dynamic atrial overdrive pacing algorithm, in AF prevention.76 The algorithm significantly increased the percentage of atrial pacing, regardless of the atrial pulse configuration and pacing site, while maintaining a slower ventricular heart rate. There was no impact on AF frequency assessed by mode switch events in either unipolar and bipolar modes in patients with sick sinus syndrome. Similar results were reported in other studies using continuous atrial pacing algorithms, with an increased (84%) prevalence of atrial pacing and significant reduction in atrial premature beats (by 79%) with no effect on symptomatic AF event frequency. However, a moderate reduction (48%) in AF episode duration was observed with a modest improvement in quality of life.77 The Biotronik (Lake Oswego, OR) closed loop system (CLS) uses a feedback mechanism to modulate its overdrive pacing algorithm. Results of a three-arm parallel comparative study of DDD(R), DDD plus atrial overdrive, and DDD plus physiological rate response algorithms showed that AT/AF burden was significantly lower with the new algorithm (20.3 ± 63.1 min/day; P < .01) versus DDD plus overdrive (56.0 ± 184.0 min/day) or DDD(R) (63.1± 113.8 min/day) modes.78
The Atrial Dynamic Overdrive Pacing Trial (ADOPT-A) randomized 399 patients with sinus node dysfunction and paroxysmal AF to DDD(R) pacing versus DDD(R) pacing plus dynamic overdrive pacing.79 Patients were monitored 1, 3, and 6 months after pacemaker insertion. The primary study outcome was symptomatic AF burden, defined for this trial as percentage of days with symptomatic AF events. The investigators reported a very modest but statistically significant reduction in symptomatic AF during follow-up. However, the absolute benefit diminished over time: 1.25% at 1 month compared with 0.36% at 6 months.80 In contrast, Menezes et al reported marked reduction in AF event rates when this algorithm was combined with atenolol and dual-site right atrial pacing. The Prevention of Immediate Reinitiation of Atrial Tachyarrhythmias (PIRAT) investigators assessed the efficacy of an early recurrence of AF suppression algorithm, with a high intervention rate of 120 beats/min. The control arm and active arm of this crossover study included the full suite of AT500 algorithms.81 No significant difference in median number of AT episodes (0.34 vs 0.37), AT/AF burden (both 1%), percentage of early recurrence of AF (28 vs 30) symptoms, or quality of life was found. In summary, the impact of novel pacing algorithms when used alone is variable, ranging from modest improvement to little or no effect. Antiarrhythmic drug therapy was not strictly controlled in many of these studies, and its impact is unknown.
Atrial Fibrillation without Concomitant Bradycardia
The PAF-PACE study enrolled 42 patients without a bradycardia pacing indication and involved a three-way crossover design comparing no pacing (OAO mode) with two atrial overdrive algorithms of different intensity.82 Medium (median, 0.88, P = .01) and high atrial overdrive (median, 0.75; P = .002) pacing reduced symptomatic AF episodes as documented by ECG versus no atrial overdrive (median, 2.03). Subgroup analysis showed that patients with a high resting heart rate also derived benefit from atrial overdrive. In a randomized crossover pilot study, 38 patients with symptomatic paroxysmal AF “without bradyarrhythmias” were randomized to atrial pacing lower rate 70 beats/min and prevention and ATP therapies ON or to atrial pacing lower rate 34 ppm and prevention and ATP therapies OFF.83 This hybrid therapy of preventive and ATP pacing and antiarrhythmic drugs may significantly reduce but not abolish AF burden if septal pacing is realized.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree