CHAPTER 69 Descending Thoracic and Thoracoabdominal Aortic Surgery
Chapter 68 on aortic arch surgery dealt with definitions and the micropathology of aortic diseases. The discussion of brain protection during deep hypothermia and circulatory arrest is also relevant to descending and thoracoabdominal aortic surgery when deep hypothermia and circulatory arrest are used. The reader is referred to Chapter 68 for a more detailed review. In this chapter, the discussion of central nervous system protection focuses mainly on protection of the spinal cord against injury during descending and thoracoabdominal aortic surgery. The etiologic and predisposing factors, preoperative workup, and operative procedures are also reviewed.
CLASSIFICATION OF DESCENDING THORACIC AND THORACOABDOMINAL ANEURYSMS
This chapter discusses the issues associated with nondissecting types of aneurysms of the descending thoracic aorta and thoracoabdominal aorta.1–59 Aortic dissection and dissecting aneurysms are dealt with in Chapters 70, 71, and 72.
Previously, descending thoracic aortic aneurysms were classified according to the extent of involvement and replacement at the time of surgery.45 In a study of 832 descending thoracic aortic aneurysms,45 this classification was used to evaluate the outcome after surgery, namely, the risk for development of a spinal cord neurologic deficit resulting in either paraplegia or paraparesis. After data collection for the analyses, the descending aorta was divided into three equal extents: extent A, the proximal third; extent B, the middle third; and extent C, the distal extent. For the purposes of statistical analyses, these extents were used to determine the incidence of neurologic deficit. The influence of replacing the entire descending aorta was also analyzed. The results of the analyses are discussed under outcomes.
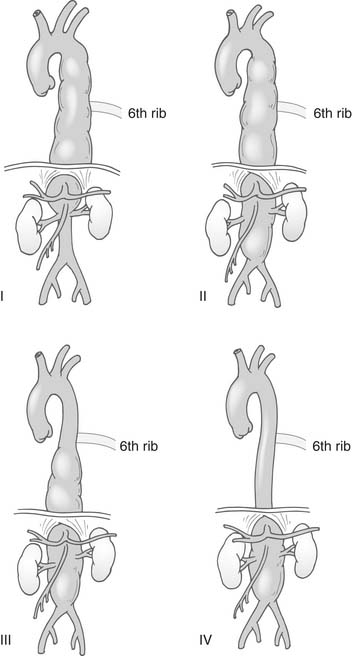
Thoracoabdominal aneurysms were classified by Crawford and others38,44,48 into four extents (Fig. 69-1). Type I thoracoabdominal aneurysms involved the descending aorta proximal to the level of the sixth rib to above the renal arteries; type II thoracoabdominal aneurysms involved the descending aorta proximal to the level of the sixth rib but beyond the renal arteries; type III thoracoabdominal aneurysms involved the distal descending aorta beyond the sixth rib and a variable extent of the abdominal aorta; type IV thoracoabdominal aneurysms involved the abdominal aorta without involvement of the descending aorta. These classifications revealed marked differences between the groups of the expected risk of neurologic deficits involving the spinal cord and, to some extent, the risk of renal failure and mortality. Type II suffered the worst outcomes. Subsequent analyses showed that the risk for development of paralysis in Crawford type I aneurysms varied according to whether the aorta was replaced below the celiac artery.48 Later, it was suggested that type III thoracoabdominal aneurysms should be further divided into a separate group according to the extent of abdominal involvement.26 These aneurysms, however, are seldom seen, and their influence on outcome was not strong enough to warrant a separate classification. Crawford type II thoracoabdominal aneurysms also have varying outcomes according to the involvement of the distal aortic arch and whether the entire abdominal aorta down to the iliacs needs to be replaced.
ETIOLOGIC AND PREDISPOSING FACTORS
Congenital Lesions
Congenital lesions of the descending aorta are fairly frequent, in contrast to the thoracoabdominal aorta, in which they are rarely observed.38 The most common congenital lesions involve the distal aortic arch and the proximal third of the descending aorta. In this area, the most frequently occurring lesion is coarctation of the aorta,38 either missed in childhood or seen in patients who have undergone previous surgery for coarctation of the aorta. Not infrequently, multiple operations have been done and patients present as adults with restenosis, an aortic replacement graft or repair that is of inadequate size for an adult, aneurysm formation proximal or distal to the previous repair, or rupture of an old previous repair including, for example, knitted grafts inserted in childhood. A lesion sometimes takes the form of an interrupted aortic arch associated with descending aorta disease. Other congenital lesions include a large Kommerell diverticulum associated with an aberrant right subclavian artery or a right-sided aortic arch (see Chapter 68). Rarely is thoracoabdominal aortic congenital coarctation (sometimes referred to as the middle aortic syndrome) seen. Some of these thoracoabdominal coarctation lesions may be related to other diseases, such as Takayasu’s disease or neurofibromatosis.38 On occasion, aneurysms may be observed in the descending or thoracoabdominal aorta and are most probably the result of chronic congenital infections, particularly from the use of intravenous or arterial cannulas that became infected. We observed this in one of our pediatric patients.38
Medial Degenerative Aneurysms
Medial degenerative aneurysms of the descending or thoracoabdominal aorta are associated with loss of elastic tissues in the aortic wall. Depending on whether cigarette smoking and chronic pulmonary disease are associated factors, there is a variable extent of atherosclerosis within the aortic wall. As aneurysms enlarge, there is an increasing amount of atheromatous material deposition and clot formation within the aneurysms. Areas of clot even appear to resemble aortic dissections on computed tomography (CT) scans or magnetic resonance imaging (MRI). Aneurysms are typically fusiform in nature, although there may be areas of weakness that have a bubble appearance on angiography or magnetic resonance angiography studies. A variant usually seen in older women with long histories of pulmonary disease and cigarette smoking is a penetrating ulcer that can lead to either dissection or, if it is successfully healed, a saccular aneurysm.38 These are most frequently seen in the distal part of the proximal third or middle third of the descending aorta.
Because of extensive atheroma and clot formation in the aneurysms, patients may present with evidence of distal embolization, such as “trash foot,” pancreatitis, abdominal angina, bowel infarction, progressive intermittent claudication, and progressive renal failure. Very rarely, patients are seen with distal embolization from atheromatic and thrombotic lesions in the arch or descending aorta without aneurysms being present. In these patients, a hypercoagulable state is usually found.38
Furthermore, those patients who have large aneurysms and a long history of medial degenerative aneurysms with extensive atherosclerosis in the aortic wall will show evidence of visceral artery lesions, such as renal artery, celiac artery, and superior mesenteric artery stenoses.42,43 During a period of time, total vessel occlusions will occur.43 Of note, CT scans should be carefully examined for both calcium and atheroma in the distal aortic arch to check if the aortic arch can be safely clamped for more proximal extending medially degenerative aneurysms. The reason for this is the obvious risk of stroke in these patients. If atheroma, clot, or calcium is found, alternative methods for doing the proximal anastomosis should be considered.
Mycotic Aneurysms and Infected Grafts
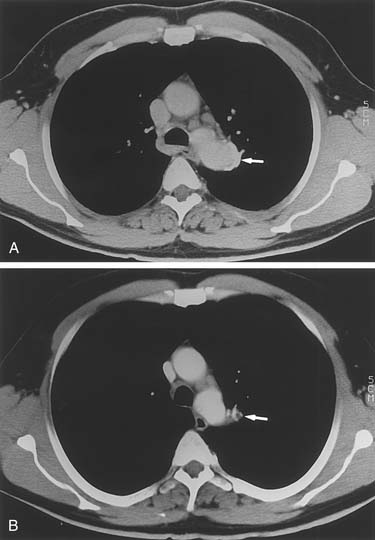
Mycotic aneurysms involving only the descending aorta are quite uncommon. More frequently, these occur either in the lesser curve of the aortic arch or, for thoracoabdominal aneurysms, in the area opposite the visceral vessels. Nevertheless, saccular aneurysms of the descending aorta can become infected, resulting in mycotic aneurysms (Fig. 69-2). Similarly, on bacterial culture of clots removed from descending or thoracoabdominal aneurysms, bacteria are often found to be growing within the aneurysms. Of interest, these bacteria appear to have little influence on the postoperative risk of graft or wound infections.
Infection in previously inserted descending or thoracoabdominal grafts can be a complicated problem to manage. The infection may or may not be associated with a left-sided chest empyema, particularly if the patient has recently undergone an operation. Diagnosis can be difficult to obtain. CT-guided aspiration of any fluid around the graft is the most accurate method of determining graft infection. Treatment options include irrigation of the cavity with antibiotics; resection of the infected graft material and placement of a new tube prosthetic graft or an allograft; and resection of the segment of the aorta and oversewing of the aortic stumps with extra-anatomic bypasses, either aorto-abdominal aorta or bilateral axillary-femoral bypasses.32,34,38,44
Traumatic Injuries of the Aorta
Traumatic injuries of the aorta are either penetrating or blunt injuries.38 Penetrating injuries require immediate attention because most patients are in shock and have lost large volumes of blood. Most injuries can be repaired by direct suture repair and usually do not require graft insertion. High-velocity missile injuries from either shrapnel or bullets are rarely survived; if the patient does survive long enough to reach the operating room, extensive destruction and secondary injury are usually found.
Blunt injuries of the aorta most commonly occur in the proximal descending aorta at the isthmus.38 Parmley and associates24 performed a study of 275 autopsies and noted that 45% were at the isthmus, 23% in the ascending aorta, 13% in the descending aorta, 8% in the transverse aortic arch, 5% in the abdominal aorta, and 6% in multiple sites. Clinically ascending aortic injuries rarely undergo operation because patients do not survive long enough to reach a hospital. Similarly, only 2% of patients undergo operation for descending aortic tears.38 In approximately one fifth of autopsies in fatal motor vehicle accidents, victims show a ruptured aorta. Multiplane angiography has been the “gold standard” for identification of tears. However, spiral CT with three-dimensional reconstruction or transesophageal echocardiography is being increasingly used for diagnosis.38
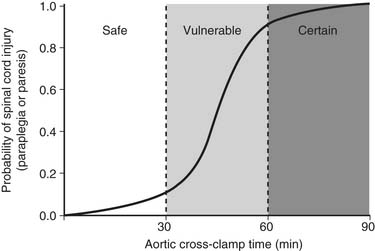
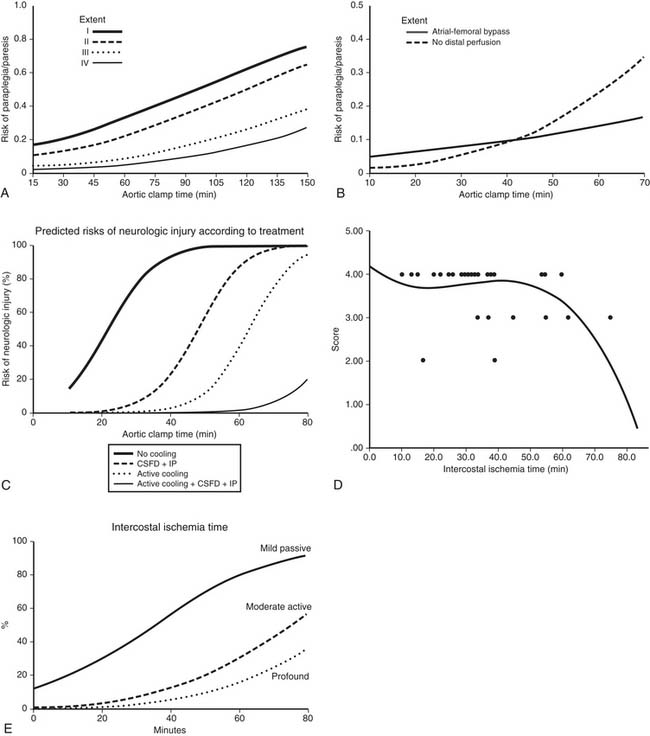
Patients with ruptured aortas are often hemodynamically unstable because of either shock or hypertension. Before surgery, while the operating room is being prepared, the patient’s blood pressure needs to be stabilized. The method to use (clamp and sew or distal perfusion with pump, shunt, or cardiopulmonary bypass) to protect the spinal cord during aortic cross-clamping is still debated. In a previous review of 596 patients,40 there were no differences in spinal cord injuries; however, mortality rate was higher with cardiopulmonary bypass. In a more recent review of 1742 patients, Von Oppell and coworkers found that cardiopulmonary bypass and distal perfusion reduced the risk of paralysis.38,58 A prospective multicenter trial reported that clamp and sew (P = .002) and an aortic cross-clamp time exceeding 30 minutes (P = .01) were associated with postoperative paraplegia.12
Available results suggest that both distal perfusion with centrifugal pump and clamp and sew can be equally safe for less than 30 minutes of aortic cross-clamping (Fig. 69-3). With increasing cross-clamp times, usually because of related, more complicated lesions and tears, the risks of paralysis and renal failure increase with both techniques, although less so with distal perfusion.12,38,39,58
Full cardiopulmonary bypass with circulatory arrest is usually reserved for complicated lesions involving the aortic arch. These lesions are best treated with circulatory arrest after the patient recovers from the initial injury and scar tissue forms so it will hold sutures. In a review of the literature40 of 44 patients initially treated with medical management and subsequently treated with elective surgery, results were excellent. Initial management of patients with traumatic rupture of the aorta associated with complicated injuries of other organ systems or of the aorta is similar to the management of acute aortic dissections of the descending aorta. Patients must be carefully observed in the intensive care unit and treated with antihypertensive medications to ensure that no leakage occurs. It is important to continue to monitor patients carefully for any evidence of leaks for the first week or two.
In patients with traumatic tears of the aorta, the classic site of tears is at the isthmus. These simple tears can often be repaired with a running suture and with the aid of pledgets. In more complicated lesions, a tube graft with a resection of the aorta with end-to-end anastomoses is required (Fig. 69-4). On occasion, aortic dissection is precipitated by trauma, and in this situation, the site of the tear is repaired. The remaining aorta, however, is managed conservatively with careful blood pressure control and follow-up, as with classic types of DeBakey III or Stanford B dissections. Rarely, chronic traumatic saccular aneurysms may resolve spontaneously over time (Fig. 69-5).28 Increasingly, we are now treating traumatic tears with stent grafts. Initial results suggest that mortality risk and risk of paralysis are at least halved.
Aortitis
There is an increased frequency of aortitis associated with giant cell arteritis in the United States and in other countries such as Iceland.38 The reason for this is unknown. In patients with giant cell arteritis of the aorta, it appears that about one third have a history of polymyalgia rheumatica. In addition, approximately 10% of patients with temporal arteritis will typically progress many years later to giant cell arteritis of the aorta.
In the United States, Takayasu’s disease is uncommon, although it is more frequent in patients with a Mediterranean family background. Takayasu’s disease of the aorta has been classified into various categories as illustrated in Chapter 68. Diagnosis is usually based on a symptom complex rather than on aneurysm type. The disease may also transition from an acute phase to a subacute phase before progressing to a chronic phase. Most aneurysms are detected at the chronic stage. Should an aneurysm form at the acute phase, we recommend treatment with steroids, but there is an increased risk of aortic dissection or rupture. In Takayasu’s disease, segments of the aorta may be skipped and spared of inflammatory lesions (see Chapter 68).38 Erythrocyte sedimentation rate and C-reactive protein levels (>1 mg/dL) are useful for following long-term risk of reoperation and activity.
Tumors
Tumors of the aorta are rare, although when they are found, they tend to occur in the descending and thoracoabdominal aorta segments. In a collected series of these patients from the literature, the prognosis has generally been poor.38 Treatment usually involves resection and graft replacement with or without chemotherapy and radiotherapy.38
Reoperations
Reoperations on the descending and thoracoabdominal aorta are increasing in frequency. For example, in a recent analysis of our patients undergoing surgery, one third had undergone previous descending or thoracoabdominal aneurysm operations, excluding patients who had had previous abdominal aneurysm repairs or ascending arch repairs.50 The reason for this is the natural course of dilatation of the aorta in other unresected segments, particularly if dissected.
The main reasons for reoperation are progression of aneurysmal dilatation of unresected segments of the aorta and either false aneurysm or saccular aneurysm formation after previous repair. In patients who have had previous acute dissection repairs of the ascending aorta, aneurysm formation in the aortic arch and descending or thoracoabdominal aorta is a common entity. Patients who have had previous descending aortic aneurysm replacements will often present with distal degenerative thoracoabdominal aneurysms below the previous descending repair. Less commonly seen are patients who either develop false aneurysms at the various anastomoses or who develop saccular aneurysms of the Carrel patches where intercostal lumbar or visceral vessels have been reattached to a new aortic graft. Albeit rare, this is a problem that should particularly be watched for in patients with Marfan syndrome. Another problem more frequently observed is in that subset of patients with previous stent grafts inserted in the descending aorta who present with complications related to the stent graft (e.g., migration, kinking, or leaks and broken stents) or aneurysm formation distal to the stent grafts. The latter problem is not surprising in that the formation of aneurysms in the aorta tends to be of a progressive nature. Because stent grafts do not limit expansion of the aorta, aneurysm formation at the anastomotic landing sites will often extend beyond the previous stent grafts.38
HISTORY AND PHYSICAL EXAMINATION
Arch aneurysms associated with descending or thoracoabdominal aneurysms involving the distal aortic arch may be manifested with associated hoarseness or respiratory problems, particularly wheezing related to compression of the left bronchus. The esophagus may also be compressed by the aneurysm at the junction of the aortic arch and the descending aorta or if the descending aorta aneurysm swings into the right side of the chest by being pinched between the vertebral bodies and the aorta. On CT scan, this may appear as a dilated proximal esophagus with fluid and results in dysphagia. Hoarseness is caused by the recurrent nerve’s being stretched by the aneurysm as the nerve wraps around the isthmus of the aorta and the ligamentum arteriosum.38
For reasons yet to be determined, smoking plays a much larger role in descending and thoracoabdominal aneurysm formation than in ascending or arch aneurysms. Tobacco addiction with patients currently smoking is a common preoperative problem in patients presenting for descending or thoracoabdominal aneurysm repair. Thus, a history of respiratory problems and a determination of respiratory capacity are essential before descending or thoracoabdominal aneurysm surgery. In a previous prospective study47 examining pulmonary function test results before thoracoabdominal surgery, there was no single cutoff point at which the risk of postoperative respiratory failure, defined as more than 5 days of postoperative ventilation, became significant. Nevertheless, at a forced expiratory volume in 1 second (FEV1) of less than 1.2 L/min, the risks increased considerably. Of course, the patient’s body habitus, including the smaller size of a woman, must be factored into this. Furthermore, if the aneurysm is relatively large, occupying much space in the left side of the chest, respiratory function may improve after resection of the aneurysm, although this is not always the case. Forced expiratory flow FEF25%-75% was found in our prospective study to be the most effective predictor of postoperative respiratory complications. The reason for this is that it gives some indication of the patient’s strength of coughing and thus the ability to clear secretions postoperatively. Also, significant carbon dioxide retention on resting blood gas analysis is a relative contraindication for surgery as is the case for patients receiving chronic supplemental oxygen therapy.38,47
During the history and physical examination, it is clearly important to establish any potential risk factors for and any history of cardiac disease. Thus, patients with atherosclerotic coronary artery disease have significantly greater risk of in-hospital mortality after these types of operations and a poorer long-term survival.38,44 In a previous study, two thirds of the late deaths were related to coronary artery disease.38,44 For this reason and the perioperative risk of myocardial infarction, the presence of coronary artery disease needs to be thoroughly examined. In addition to checking for coronary artery disease, any valvular disease, particularly aortic valve regurgitation, needs to be investigated because during aortic cross-clamping, even with atriofemoral bypass, there is a significant increase in cardiac afterload. If this is associated with aortic valve regurgitation, the patient may go into acute heart failure from myocardial distention. The patient’s left ventricular muscle strength and ejection fractions are also strong determinants of early and late outcome after surgery. Part of the reason for this is that the increased afterload presented to the left ventricle results in a temporary left ventricular dysfunction after surgery, and if the patient has a poor ejection fraction, both the early and late mortality rates are increased. In a study of 132 patients undergoing descending or thoracoabdominal repairs, an interesting finding was that patients who had modest coronary artery disease and did not have coronary bypasses or stents had a higher risk of myocardial infarction and poorer long-term survival than did those who had bypass surgery. This could be in keeping with our current understanding of coronary disease.
A careful search for any renal disease should also be done.42 An increased preoperative creatinine level in the blood has a strong correlation with both operative and late postoperative outcomes. Indeed, it is one of the most important risk factors for both early and late mortality by multivariable analyses.38,42–44 Furthermore, if creatinine levels are significantly elevated, evidence for renal artery stenosis should also be investigated.
PREOPERATIVE TESTING
Pulmonary Function Tests
As indicated before, all patients undergo pulmonary function tests before surgery.47 If the patients are actively smoking and are scheduled to undergo a pulmonary function test, an effort is made to wean the patients off their tobacco dependency and to improve their pulmonary function before surgery. Patients who are actively smoking and undergo thoracoabdominal descending aortic surgery are more prone to prolonged intubation and bronchial secretions after the operation that will complicate their postoperative recovery.
SPINAL CORD PROTECTION
Both the pathophysiology and etiologic factors causing paralysis after descending and thoracoabdominal aneurysm surgery have been extensively reviewed elsewhere.27,36,38,49,50 Briefly, based on both animal and human studies, there are three main mechanisms by which postoperative neurologic deficits and paralysis can arise. First, duration and degree of ischemia during the period of aortic cross-clamping are important. In patients with coarctation of the aorta, there is an extensive collateral network of blood to the spinal cord, so the degree of ischemia is not as severe as in a patient with traumatic rupture of the aorta or acute dissection in which no collaterals have been established to the spinal cord.38,39 In an extensive aneurysm (such as Crawford type II thoracoabdominal aneurysms), the degree of ischemia is more severe because of a greater amount of interference to the spinal cord blood supply. Multiple studies have shown that the duration of ischemia is an important factor. The best way of showing this is by logistic regression analysis of the aortic cross-clamp time or the intercostal ischemia time versus the risk of neurologic deficit (Fig. 69-6; see also Fig. 69-3). The relationship is an S-shaped sigmoid curve, with the risk of paralysis rapidly increasing after 30 minutes of aortic cross-clamping in patients with acute dissection or traumatic rupture of the aorta.38,39 Of interest, in patients with thoracoabdominal aneurysms, because of the extensive collateral blood supply that has been established, the curve is not quite as steep.38,44 Similarly, for descending aortic aneurysms, when there is more direct blood supply below the repair or collateral blood supply, the risk of paralysis after 30 minutes is less. Thus, research shows that the curve is moved to the right by interventions that successfully reduce the risk of spinal cord injury. During the past 2 decades, interventions have successfully reduced the risk of this aspect of spinal cord injury.8,26,27,49,50
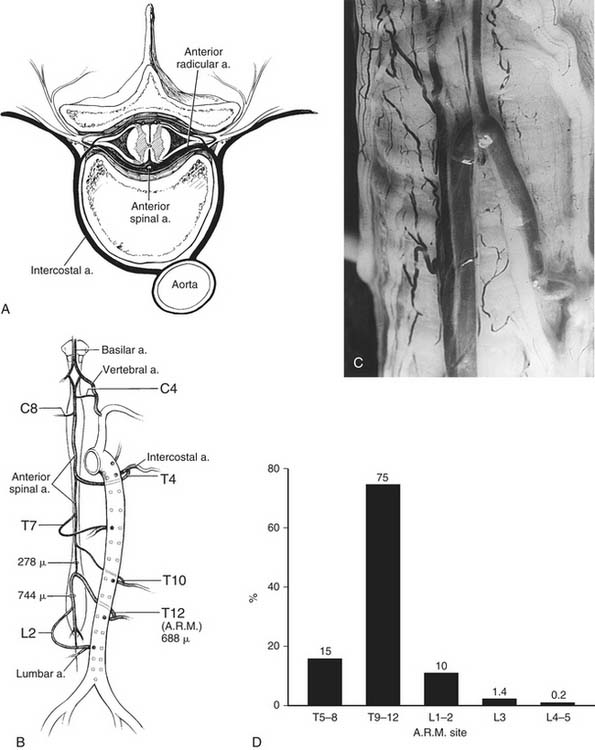
Second, failure to reestablish spinal cord blood flow after clamping of the aorta increases the risk of spinal cord injury. Thus, both animal and human studies have shown that intercostal and lumbar arteries supplying the spinal cord need to be reattached to reduce the risk of postoperative spinal cord and neurologic deficits.27,33,36,38,46,48,52
Third, after the initial ischemic event to the spinal cord, secondary injury can be related to postoperative hemodynamic instability in the intensive care unit after surgery, or the complex biochemical cascade that is set in motion by the initial ischemic injury can result in secondary reperfusion injury, including the development of apoptosis. This complex cascade of multiple pathways of events has been extensively reviewed in detail.35,38,56
Based on the pathophysiology involved in causing neurologic deficits, operative techniques and both experimental and intraoperative maneuvers have been developed to reduce the risks for development of postoperative neurologic incidents.38,44,49,50 Indeed, to address the first etiologic factor (i.e., degree of ischemia and duration of ischemia), certain intraoperative techniques are used. To reduce the risk of ischemia, every attempt is made to shorten both the aortic cross-clamp time and the intercostal ischemia time. Recently, we showed50 the intercostal ischemia time to be a more important variable than total aortic cross-clamp time with respect to neurologic injury. Therefore, quick and efficient anastomoses must be performed during the operation. We recommend the use of techniques that shorten the aortic cross-clamp time, for example, second-stage elephant trunk procedures, whenever indicated. A method we developed in 1992 based on animal experiments is a segmental sequential repair that reestablishes spinal cord blood flow.37,39,49,52 Once proximal anastomosis has been accomplished, the proximal descending aortic and subclavian clamps are removed to reperfuse the subclavian artery and to improve blood flow through vertebral arteries and the posterolateral spinal arteries. The mid descending aorta is cross-clamped during the initial cross-clamping as frequently as possible so that the remaining portion of the aorta below is perfused by the atriofemoral bypass circuit. The intercostal vessels in this segment, down to T6, are oversewn with a 1-0 suture. This segmental level is conveniently in line with the sixth rib, which has been resected during the initial thoracotomy. Next, the aorta is opened down to the clamp placed immediately above the celiac artery, and the intercostal arteries, between T6 and the celiac artery, including any upper lumbar arteries, are then reanastomosed to the graft. If possible, the graft is then de-aired and the intercostal Carrel patches are reperfused, reestablishing further blood flow to the spinal cord through the thoracic radicular arteries so that these collateral vessels can perfuse the anterospinal artery both up and down the length of the spinal artery. After this, the visceral segment of the aorta is repaired, including any lumbar arteries that need to be reattached. This segment is also reperfused both to increase spinal cord blood flow and to reestablish visceral blood flow. In Crawford type II and III thoracoabdominal aneurysms, the lumbar arteries in the abdominal segment are reattached to reperfuse them as well as the median central artery if it is present. Hence, this sequential segmental technique minimizes the period of ischemia to the spinal cord. Both our studies and those by others38,49,50,52 have confirmed that this approach reduces the risk of spinal cord injury. In addition, we have found that cooling of the patients before aortic cross-clamping, either to moderate hypothermia levels, particularly between 30° and 32° C with an atriofemoral bypass circuit,49 or to profound hypothermia levels with cardiopulmonary bypass, reduces the risk of spinal cord injury.50 Both methods appear to be equally effective in protecting the spinal cord.
Other methods of local cooling of the spinal cord include injection of cold solution into the occluded aorta, intrathecal cooling, epidural cooling, and intrapleural cooling alongside the vertebral bodies. Most of these techniques have been shown to be protective in animal studies, but human studies have shown variable degrees of success.2,4,38,46,50
To address the protection of the spinal cord and the influence of segmental intercostal or lumbar arteries, it is important to briefly review the anatomy of the spinal cord (Fig. 69-7).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree