Fig. 20.1
Platelets and the two types of granules they contain. Alpha granules contain insulin-like growth factor 1 (IGF-1), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-B), platelet factor 4 (PF4), von Willebrand Factor (vWF), thrombospondin, and fibronectin. Dense granules contain ADP, ATP, calcium, and serotonin. Both types of granules are necessary for the coagulation cascade to function correctly
Disruption of primary hemostasis such as in disseminated intravascular coagulopathy (DIC); drug-induced reactions with quinidine, quinine, vancomycin, or gold salts; bone marrow suppressed states; cardiopulmonary bypass; and alcohol toxicity leads to the inability to form an enduring clot and presents as mucocutaneous bleeding. Defects in primary hemostasis can be diagnosed with platelet aggregation assays, von Willebrand Factor functional assays, and a platelet function analyzer (PFA) [52].
After formation of the platelet plug, secondary hemostasis is initiated with the coagulation cascade to form a stable fibrin plug. Both an intrinsic and extrinsic pathway are available to initiate secondary hemostasis (Fig. 20.2); this dual pathway ensures that even patients with defects in primary hemostasis eventually develop some form of enduring clot over time (Fig. 20.3) [53]. The most important pathway in secondary hemostasis is the extrinsic pathway, which relies upon the transmembrane receptor tissue factor (TF).
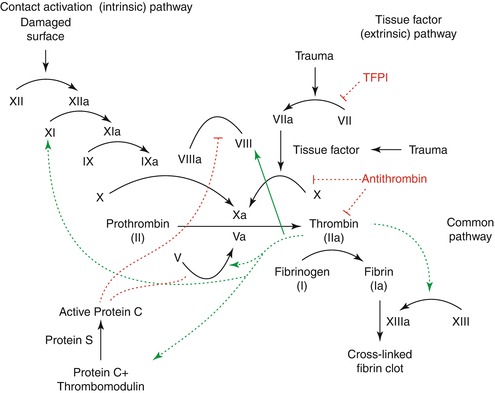
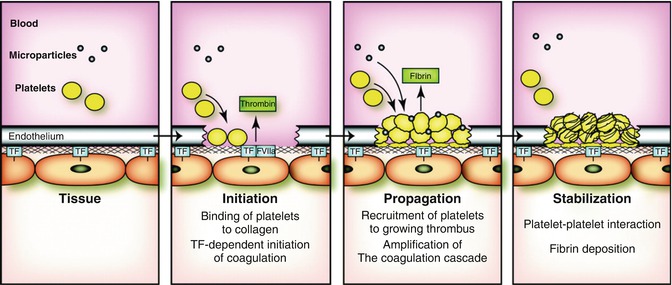

Fig. 20.2
The intrinsic and extrinsic pathway of coagulation with the interplay between the various types of factors demonstrated. Illustration by Joe Dunckley. Used in accordance with the GNU Free Documentation License Version 1.2

Fig. 20.3
Formation of a clot at the site of blood vessel injury. In a healthy individual, TF expressed by vascular smooth muscle cells, pericytes, and adventitial fibroblasts in the vessel wall is physically separated from its ligand FVII/FVIIa by the endothelium. Vessel injury leads to the rapid binding of platelets to the subendothelium and activation of the coagulation cascade by TF. Propagation of the thrombus involves recruitment of additional platelets and amplification of the coagulation cascade by the intrinsic pathway, and possibly by TF-positive MPs and TF stored in platelets. Finally, fibrin deposition stabilizes the clot. De novo synthesis of TF by platelets may also play a role in stabilization of the clot
Tissue factor is found within vascular endothelium, adventitia, brain, lung, heart, and placenta [54–61]. While deficiencies in factors VIII and IX (hemophilia A and B, respectively) have been identified, the absence of tissue factor is typically incompatible with life [62]. Constitutively, low production of tissue factor is associated with spontaneous hemorrhage in the heart, lung, and placenta [60].
Tissue factor is normally unmasked from the vascular wall following trauma. However, this factor is expressed by monocytes and possibly neutrophils and may initiate thrombosis in patients with disseminated intravascular coagulation [63–65]. Tissue factor expression upon neutrophils has been implicated as one of the causes of autoimmunity-based hypercoagulable disorders such as antiphospholipid antibody syndrome [65].
20.3.3 Extrinsic Pathway
Trauma initiates a process whereby tissue factor interacts with factor VII, forming an activated complex and beginning the cycle of thrombosis. This activated complex leads to the activation of factor X and the beginning of the final common pathway that both the extrinsic and intrinsic pathways share, discussed below.
The extrinsic pathway has a number of strict controls to prevent runaway thrombosis. This is necessary as factor VII is one of the most common factors in circulation and the lack of strict regulation would lead to rapid propagation of clot [66]. The tissue factor-factor VIIa complex is inhibited by tissue factor pathway inhibitor (TFPI), a single chain polypeptide that also inhibits factors Xa and IIa (thrombin).
20.3.4 Intrinsic Pathway
While the extrinsic pathway seems critical for thrombosis, defects in the intrinsic pathway are associated with delayed clot formation with arterial injury. The primary importance of the intrinsic pathway therefore appears to be with arterial thrombosis; clot formation throughout the veins and much of the rest of the body appears to be controlled by the extrinsic pathway [53, 59–61]. Indeed, elevated levels of factor XI have been found to be associated with increased risk of myocardial infarction and an independent risk factor for stroke [67, 68].
Contact activation initiates the intrinsic pathway when factor XII binds to an appropriately charged surface, typically the site of some sort of injury [69]. This initiates a cascade reaction where a series of trypsin-like enzymes amplify the reaction and promote thrombosis. Factor XII is activated, leading in turn to the activation of factor XI. These factors work together to activate factor IX; a deficiency of this factor leads to hemophilia B (discussed later). Factor IXa binds to activated factor VIII to initiate the activation of factor X and the beginning of the final common pathway; defects in factor VIII lead to hemophilia A. Of note, independent activation of factor XI can also occur, as seen in patients with defects in factor XII (Hageman factor deficiency) [70].
20.3.5 Final Common Pathway
The final common pathway begins with factor Xa and is regulated by factor V. The activation of factor V is inhibited by protein C; the activation of protein C is modulated by protein S. Therefore, protein C and S serve as inhibitory mediators for coagulation and their deficiency leads to a hypercoagulable state; this is seen in warfarin administration without prior anticoagulation with heparin. Protein C and S are vitamin K-dependent factors with short half-lives and are downregulated within the first 24–48 h of warfarin administration. This leads to a transient hypercoagulable state while the other factors are still being downregulated to sufficient levels [71].
Activated factors X and V lead to the conversion of prothrombin (factor II) to thrombin (factor IIa). Thrombin leads to feedback regulation by stimulating the production of protein C and thrombomodulin. Thrombin serves to convert fibrinogen to fibrin, leading to the deposition of the hemostatic plug. Cross-linking of the fibrin clot occurs with the action of factor XIII, the production of which is also upregulated by thrombin. Organization of this blood clot occurs via plasmin-mediated fibrinolysis.
Proper functioning of the coagulation cascade requires the factors discussed earlier, along with adequate concentrations of calcium and vitamin K. Calcium is a cofactor that is required for the activation of various factors; its deficiency is associated with coagulopathy [72]. Vitamin K is required for the synthesis of factors II, VII, IX, and X, and proteins C and S. A third protein, protein Z, is also regulated by vitamin K; this protein appears to play a role in degradation of factors Xa and XI [73]. Defects in protein Z have been associated with hypercoagulable disorders [74].
20.3.6 Regulation
Regulation of the coagulation cascade relies on a variety of mediators. Protein C and S inhibit the production of factor Va, leading to cessation of further thrombin production. Protein C and S production are upregulated by thrombin, leading to a feedback inhibition reaction at the level of the final common pathway. Tissue factor pathway inhibitor inhibits tissue factor, temporizing clot formation at the extrinsic pathway level. Prostacyclin (PGI2) leads to the production of adenylyl cyclase and cAMP production by platelets, leading to sequestration of calcium and general inhibition of coagulation.
20.3.7 Thrombolytics
Two other complex systems help to control coagulation: the first is the thrombolytic system, which involves plasmin and tissue plasminogen activator (tPA), and the second involves a group of inhibitors of the coagulation factors, including antithrombin III, protein C, and protein S.
The thrombolytic system dissolves fibrin clots using the serine protease plasmin. Plasmin is formed from its inactive precursor, plasminogen. Interestingly, plasmin is also activated by thrombin, which thereby limits its own clot-forming ability.
The second anticoagulant system is made up of antithrombin III and proteins C and S. Antithrombin breaks down factors IXa, Xa, Xia, and XIIa and thrombin, leading to inhibition of coagulation at both the intrinsic and final common pathways, and its activity is enhanced up to 2,000-fold by heparin.
20.4 Monitoring
There are several general tests of clotting factors commonly used to measure overall function of the coagulation cascade. The activated partial thromboplastin time test (PTT) measures the intrinsic pathway and will be increased in deficiencies of factors VII, IX, XI, XII, von Willebrand fibrinogen. PTT testing is used to monitor heparin efficacy in patients on heparin drips. The prothrombin time test (PT) measures the extrinsic pathway and will be abnormal in deficiencies of factors II, V, VII, and X and fibrinogen. Because of variations in PT level reporting, the international normalized ratio (INR) was developed to allow comparison of levels across laboratories. PT/INR testing is used to monitor vitamin K and warfarin efficacy.
The coagulation cascade is a complex interplay between dozens of proteins and cells. Its seamless function is required to avoid coagulopathies and hypercoagulable states. The division into an intrinsic and extrinsic pathway plays unique roles in coagulation and offers scientists numerous targets to deal with disorders in coagulation.
20.5 Anatomic Considerations
Superficial venous thrombophlebitis (STP) refers to thrombosis occurring in the superficial veins. Patients with STP usually present with a painful, red, tender superficial vein. Ultrasound should be performed to delineate the extent of thrombosis and rule out concomitant DVT. Concomitant PE is also not uncommon and should be ruled out. Extensive STP above the knee, especially in the great saphenous vein, or STP near saphenofemoral or saphenopopliteal junctions, merits anticoagulation along with compression or even surgical saphenofemoral disconnection. Less extensive STP can be treated with nonsteroidal anti-inflammatory medicines and compression therapy. Close follow-up is important, since STP can progress to DVT or PE.
External venous obstruction in the pelvis and the thoracic outlet lead to acute and chronic venous thrombosis. In the pelvis, May-Thurner syndrome occurs due to sustained compression of the left iliac vein by the right iliac artery. Treatment mandates removal of the external forces through iliac stenting, and several series have shown improvements in intermediate quality of life following this type of intervention. In the thoracic outlet, Paget-Schroetter syndrome occurs when the subclavian vein becomes obstructed due to repeated trauma from adjacent bony, muscular and ligamentous attachments. While management of this entity is controversial, most practitioners agree that thrombolysis of subclavian clot and either selective or routine surgical decompression of the thoracic outlet is necessary for the optimal outcomes.
20.6 Presentation
Patients with DVT usually present with acute, unilateral extremity swelling or pain. Tenderness is common on examination, but Homan’s sign (passive dorsiflexion of the foot produces calf pain) is unreliable. Redness and localized warmth are sometimes found. Differential diagnoses include cellulitis, Baker’s cyst, lymphangitis, lymphedema, and local injuries.
Patients with pulmonary embolism usually present with shortness of breath or chest pain. The chest pain can be pleuritic or unilateral. The diagnosis less commonly presents, but should be considered, in patients with syncope, shock, or cardiopulmonary arrest. Other symptoms include cough, hemoptysis, anxiety, and sweats. Signs include tachypnea (respiratory rate over 16 breaths per minute) and tachycardia (heart rate over 100 beats per minute). Unexplained hypoxia should prompt aggressive investigation for PE. The differential diagnosis for each of these symptoms is large, but common alternative diagnoses are acute myocardial infarction, aortic dissection, acute pericarditis, chronic obstructive pulmonary disease, asthma, congestive heart failure, and pneumonia.
20.7 Diagnosis
Many episodes of DVT and PE present with occult symptoms. Clinical scores have been developed to assist in diagnosis of both DVT and PE. A variety of risk assessment models exist for calibrating the risk of DVT formation and subsequent PE [43–50]. Among these various models, the Wells score stands out for its simplicity and applicability as a risk evaluation model for DVT and PE (Table 20.1). A score of two or higher indicates a significant risk for DVT, while a score less than two indicates that the risk of DVT is low. Patients with a risk of DVT should be evaluated with noninvasive imaging [51]. In the PE score, a total of 4 or less points means the risk for PE is low. When considering the diagnosis of PE, most patients receive an EKG and chest X-ray, tests useful mostly to exclude other diagnoses. However, EKG findings often consistent with PE include right heart strain and right heart block. Unexplained hypoxia also suggests PE.
Table 20.1
Clinical model for predicting the pretest probability of deep-vein thrombosis
Clinical characteristic | Score |
|---|---|
Active cancer (patient receiving treatment for cancer within the previous 6 months or currently receiving palliative treatment) | 1 |
Paralysis, paresis, or recent plaster immobilization of the lower extremities | 1 |
Recently bedridden for 3 days or more, or major surgery within the previous 12 weeks requiring general or regional anesthesia | 1 |
Localized tenderness along the distribution of the deep venous system | 1 |
Entire leg swollen | 1 |
Calf swelling at least 3 cm larger than that on the asymptomatic side (measured 10 cm below tibial tuberosity) | 1 |
Pitting edema confined to the symptomatic leg | 1 |
Collateral superficial veins (nonvaricose) | 1 |
Previously documented deep-vein thrombosis | 1 |
Alternative diagnosis at least as likely as deep-vein thrombosis | −2 |
D-dimers are a product of fibrinolysis that are often elevated in acute VTE. The D-dimer test is sensitive (85–95 %) but nonspecific (40–65 %) for acute VTE. Enzyme-linked immunosorbent assays and some immunoturbidimetric D-dimer tests are more sensitive than other methods, such as whole-blood and quantitative latex agglutination assays. Patients with a low risk DVT score and a negative D-dimer have less than a 1 % chance of having DVT [15]. Similarly, a low risk PE score and a negative D-dimer carries a less than 1 % risk of nonfatal PE [16]. These DVT and PE patients rarely need further testing (Table20.2).
Table 20.2
Pulmonary embolism score
Variable | Score |
|---|---|
Clinical signs and symptoms of DVT (minimum of leg swelling and pain with palpation of deep veins) | 3.0 |
Alternative diagnoses less likely than PE | 3.0 |
Heart rate >100 beats per minute | 1.5 |
Immobilization >3 days or surgery in the previous 4 weeks | 1.5 |
Previous PE or DVT | 1.5 |
Hemoptysis | 1.0 |
Malignancy and receiving treatment, treated in the last 6 months, or palliative | 1.0 |
Duplex ultrasonography is the accepted standard for noninvasive venous imaging in suspected DVT. Ultrasound for thigh DVT has sensitivity of 97 %, specificity of 86 %, positive predictive value of 87 %, and negative predictive value of 97 % for evaluation of the femoropopliteal system. Accuracy from ultrasound is operator dependent. Small calf vein thrombi can be missed on ultrasound. For this reason, patients with continued suspicion of DVT and an initially negative ultrasound study should be rescanned in around a week to rule out a significant thrombus extension from an initially missed calf vein thrombus. Magnetic resonance venography (MRV) is an alternative to ultrasound useful for evaluation of pelvic and caval thrombi. Wide adoption of MRV is limited by rare but devastating cases of nephrogenic systemic fibrosis (NSF). In the future, additional contrast agents and technical refinements will permit increased use of magnetic resonance (MR) in the evaluation of VTE, particularly in cases of suspected central stenoses.
Computed tomographic angiography (CTA) diagnoses PE with a high sensitivity and specificity, enabling rapid diagnosis with minimal morbidity. The test is contraindicated in the setting of renal insufficiency due to the relative nephrotoxicity of the more than 100 mL of contrast required for the study [75]. Since the 1960s, pulmonary angiography has been the invasive gold standard for diagnosing emboli to the pulmonary vasculature. The procedure carries morbidity of approximately 6 %, related primarily to access complications, and mortality of less than 0.5 %, related to ventricular dysrhythmias due to right heart catheterization. Ventilation perfusion (V/Q) scans are another alternative but are insensitive and nonspecific and useful only if pretest clinical probability matches V/Q scan result.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


