Chapter 93 Curative Catheter Ablation for Supraventricular Tachycardia
Techniques and Indications
Accessory Atrioventricular Connections
The anatomic substrate of accessory AV connections is the myocardium bridging the AV annuli, which, in normal individuals, are fibrous and electrically insulating (Box 93-1).1 The sequence of normal initial ventricular septal depolarization is altered by conduction through these connections inserting into the ordinary myocardium and bypassing the normal insulated and septally conducting His-Purkinje system. The relatively slow spread of activation through the ordinary myocardium contrasts with the coordinated septal endocardial breakthrough of Purkinje ramifications and results in the δ-wave in the surface electrocardiogram (ECG). In addition to providing an additional route for impulse conduction between the atria and the ventricles, nearly all accessory connections exhibit conduction properties different from the AV node. Decremental conduction is not ordinarily seen; that is, with increasing frequency or shortening coupling intervals, the conduction time across the pathway does not significantly increase.
Box 93-1 Checklist for Catheter Ablation of Accessory Atrioventricular Connections
Setup
Evaluation
ECG, Electrocardiogram; IVC, inferior vena cava; AV, atrioventricular; VA, ventriculoatrial; SVT, supraventricular tachycardia; AVNRT, atrioventricular nodal re-entrant tachycardia; AP, accessory pathway; RF, radiofrequency.
Electrophysiological Characteristics of Accessory Pathways
During sinus rhythm, ventricular extrastimuli resulting in atrial activation preceding retrograde bundle of His activation indicate an accessory connection. If moving the ventricular pacing site from the apex toward the septum decreases the stimulus to atrial activation time instead of increasing it, an accessory VA connection should be considered. Moving away from the apex increases the conduction time to the normal AV conduction system through the distal Purkinje myocardial interface, whereas it decreases the conduction time to the annular insertion of an accessory pathway.2 Similarly, high output–dependent capture of the insulated right bundle or the bundle of His contrasted with lower output ventricular myocardial capture at the same site can show changes in atrial activation sequence, retrograde His to atrial activation time, and stimulus to atrial activation timing, which suggest the presence of more than one retrograde pathway of VA conduction.3 Unchanged atrial activation sequence coupled with a constant H-A interval and prolongation of the stimulus-A interval resulting from loss of His–right bundle capture indicate the presence of the normal VA conduction alone. Conversely, the absence of change in any of the intervals and sequences indicates the sole presence of accessory pathway retrograde conduction. If the accessory pathway is remote from the pacing site or is captured only with a long conduction time or if conduction through the AV node is very rapid, conduction through the accessory pathway may be completely masked. In practice, left free wall pathways remote from a right ventricular pacing site may fulfill these conditions and are therefore likely to be masked.
During a tachycardia, evidence of conduction through an accessory AV connection can be obtained by delivering late ventricular extrastimuli coincident with or 10 ms before activation of the bundle of His, thus ensuring the encountering of complete refractoriness within the bundle of His. If the extrastimulus is earlier than the His electrogram, the lack of anticipation of the ventricular electrogram, the bundle of His electrogram, or both confirms His-Purkinje refractoriness. The presence of conduction through an accessory connection is indicated if the ventricular extrastimulus advances or delays atrial activation or terminates the tachycardia without conduction to the atria.4 Tachycardia termination by a His-synchronous ventricular extrastimulus without conduction to the atria or with anticipation of the succeeding ventricular or bundle of His electrogram indicates participation of the accessory pathway in the tachycardia.
In addition to establishing the presence of an accessory connection, the electrophysiological study (EPS) allows assessment of the arrhythmogenic potential of the accessory connection. The indications for curative ablation of accessory pathways chiefly depends on their proven threat—pre-excited AF degenerating to VF—or their potential threat, indicated by R-R intervals shorter than 200 to 250 ms during AF or the presence of clinical tachycardias using the accessory pathway.5
Electrophysiological Localization
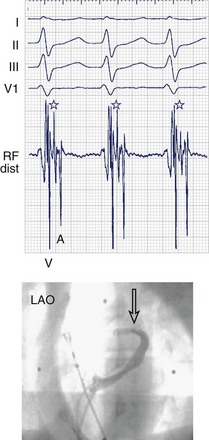
When even the best unipolar and bipolar endocardial electrograms are not good enough, an epicardial or intramyocardial pathway insertion may need to be evaluated or considered. Ventricular electrograms close to or at the site of insertion can be late, not only because the insertion may be far from the endocardium but also because of the endocardial insertion of an oblique pathway. Changing the pacing site (e.g., from the right ventricular apex to the lateral left ventricular or the right ventricular infundibulum) can help distinguish apparently early atrial electrograms (during ventricular pacing) because of an oblique pathway course. Simultaneous comparison of endocardial and epicardial recordings obtained from within the coronary sinus is useful; bracketing, as well as electrogram timing and dv/dt (rate of ventricular electrogram depolarization) comparison, can provide valuable clues. Ablation within the coronary sinus may be necessary (Figure 93-1), although conventional RF delivery achieves only low power and is frequently ineffective. Ablation in the coronary sinus and veins with a catheter with an open irrigated tip can achieve good results; however, stepwise increments in RF power (a cautious maximum of 25 W) are prudent. Pops in the thin-walled coronary venous structure can be devastating; damage to adjacent coronary arteries has also been reported.
Local Electrogram Characteristics
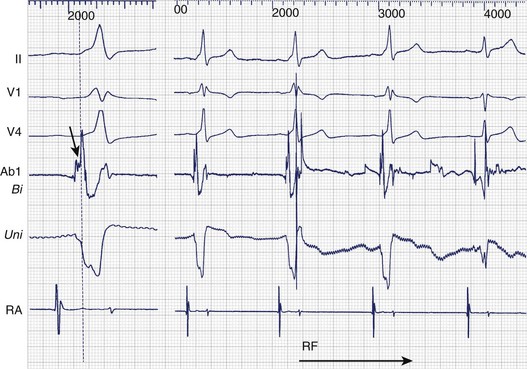
Bipolar and unipolar electrograms should both be used for mapping (Figure 93-2)—the former because of their higher signal/noise ratio and the latter because of their simple morphologic pattern recognition–based analysis.6 Localization based on bipolar electrograms requires distinction of atrial electrograms from ventricular electrograms by using late-coupled ventricular and atrial extrastimuli. However, these maneuvers can be difficult to perform or analyze and may even induce arrhythmias. The contribution of the proximal ring electrode to bipolar electrograms from the distal bipole can be misleading. Atrial electrograms can be distinguished from ventricular electrograms by using unipolar electrograms from the distal electrode.
Unipolar electrograms with a steep QS morphology are particularly useful for localizing the site of ventricular insertion on the basis of a steep QS morphology (the absence of an initial R wave) during sinus rhythm, pacing, or even ongoing AF and also in patients with Ebstein’s anomaly who exhibit low-amplitude, fractionated bipolar electrograms on the tricuspid annulus. Unipolar electrograms should be recorded with wide band filters—0.05 to 500 Hz—because the low-frequency content makes important contributions to the generation of RS or QS patterns. Instead of Wilson’s central terminal, a remote cutaneous or inferior vena cava (IVC) electrode may be useful as a ground, allowing common mode rejection of contaminating 50- or 60-Hz line noise. Notch filters should also be used with caution, if at all. Once the atrial and ventricular electrograms have been recognized, the intervening deflections represent presumptive accessory pathway potentials (see Figure 93-1).7 Certainly, the best validation is the prompt abolition of accessory pathway conduction by RF ablation at this site (assuming appropriate power delivery and contact). In practice, accessory pathway potential validation often is a retrospective exercise.
Individual Pathway Locations
Septal Atrioventricular Accessory Connections
The main concern with regard to the mid-septal pathways is to avoid damage to the AV node and the normal conduction axis. As for the para-Hisian pathways, proximity to the bundle of His allows an estimation of this risk. Proximity to the compact AV node is, however, difficult to estimate in the absence of an electrogram marker. The appearance of junctional rhythm is a clear warning that should prompt cessation of RF delivery, and a narrow QRS complex without a preceding P wave should not be mistaken for loss of pre-excitation. Using conventional RF, the strategy for pathways estimated to be close to the AV node or the bundle of His should center around careful mapping for the best electrograms and delivering low RF power at sites thought to be farthest from the conduction axis, usually on the ventricular side of the AV ring. At prospective ablation sites, it is useful to verify the presence and the amplitude of a bundle of His deflection concealed by pre-excitation by using programmed stimulation to induce antegrade pathway block or sustained orthodromic AVNRT. RF power may be increased cautiously in steps of 5 W, but energy delivery should be terminated immediately in case of junctional rhythm or if loss of pre-excitation does not occur promptly. Cryoablation offers the theoretical advantage of reversible cryomapping. In practice, although a greater margin of reversible lesion creation with cryoablation and therefore a lower risk of AV block may exist, this energy source has a clearly higher risk of recovery of pathway conduction.8
The posteroseptal pathways have a higher likelihood of an epicardial course or insertion. Moreover, the anatomic boundaries of the posterior pyramidal space frequently require a choice to be made between the right or left endocardial sites and the sites within the proximal coronary sinus or the middle cardiac vein. A steep QS complex in lead II or an rS complex in leads V5-V6 during pre-excitation may be a clue to an insertion into the middle cardiac vein.9 If endocardial mapping is not good enough or the ablation is unsuccessful, mapping within the coronary sinus and the middle cardiac vein is performed under the guidance of a coronary sinus angiogram. Occlusion balloon angiography provides the best opacification of the great cardiac vein and related branches, but adequate visualization of the proximal coronary sinus and the middle cardiac vein can be achieved from the femoral approach by using an Amplatz catheter. Successful ablation sites are frequently clustered in proximity to venous anomalies such as aneurysms or diverticula. A superior approach from the internal jugular vein provides a relatively straight and vertical catheter course to the middle cardiac vein and should be considered in case of difficulty with the femoral approach. Ablation within the coronary sinus or cardiac veins with a conventional nonirrigated ablation catheter is frequently ineffective because of low delivered powers and high electrode temperatures as a consequence of limited blood flow around the electrode. Ablation in the middle cardiac vein can damage the posterior descending and posterior left ventricular branches of the distal right coronary artery. Ineffective low power delivery can be overcome by using an irrigated tip catheter that allows power to be titrated up to a limit of 25 to 30 W to avoid pops or damage to nearby coronary arteries (within 2 to 3 mm of the site of ablation).
Specific Situations
The substrates of the “Mahaim” pathways (decremental atriofascicular or atrioventricular pathways) and permanent junctional reciprocating tachycardias are both thought to be accessory pathways with long conduction times—antegrade in case of the atriofascicular or AV Mahaim pathways and retrograde in case of persistent junctional reciprocating tachycardia (PJRT). In addition, these two accessory pathway variants share another characteristic—that of one-way conduction only. The few available histologic studies suggest that the anatomic substrate of PJRT is a long and tortuous muscular fascicle, whereas in the case of the Mahaim fiber, an accessory node–like structure is thought to exist at its atrial origin.10 Atriofascicular and AV Mahaim fibers are most effectively ablated by targeting the pathway potentials at the level of the annulus; they resemble the bundle of His potentials but continue to precede ventricular activation even during pre-excitation. PJRT is ablated by targeting the earliest atrial activation during the tachycardia, and, as with every ablation within that posteroseptal area, care must be taken to ensure a reasonable distance from the normal AV conduction axis.
An accessory pathway with a large insertion or an insertion with multiple branches may occasionally be encountered.11 Multiple coalescent lesions, each of which modifies local electrogram parameters, have been used.
Another uncommon variant is an appendage to the ventricular connection characterized by an insertion bridging the appendage tip to the ventricle away from the annulus.12 Careful mapping, aided by three-dimensional mapping as needed, can clarify the exact location of the insertion. Similarly, the unusual variant of surgically acquired pre-excitation is encountered rarely after right atrial appendage anastomosis to the right ventricular outflow tract (RVOT) (historically performed as a palliative procedure for tricuspid atresia). In the appropriate surgical context and with the pre-excited QRS resembling an RVOT tachycardia, careful mapping has allowed successful ablation.
An additional arrhythmia substrate such as AVNRT or AT may coexist. The electrophysiological maneuvers described above can assist in deciding whether the accessory pathway participates in the tachycardia.13 However, in practice, elimination of the accessory pathway substrate typically unmasks the AVNRT or AT, which can then be ablated in the standard fashion.
Indications for catheter ablation include the following:
Atrioventricular Nodal Re-entrant Tachycardias
AVNRT is the result of a re-entry circuit in the AV junctional region (Box 93-2), although debate about anatomic delimitations continues. The functional heterogeneity of AV junctional tissues, primarily with respect to conduction velocity and refractory periods, permits the sustenance of an excitable gap re-entry circuit. Because of anatomic factors and the lack of distinct electrophysiological markers of activation, it is difficult to delineate the anatomic extent of the circuit. Nevertheless, available evidence suggests that the peri-nodal atrium, the compact AV node, and possibly a part of the proximal bundle of His are involved. Although no significant anatomic abnormalities have been found in patients with AVNRT, multiple posteriorly situated pathways or approaches to the AV node have been described.14