Chapter 15 Coronary Artery Disease Detection
Pharmacologic Stress SPECT
INTRODUCTION
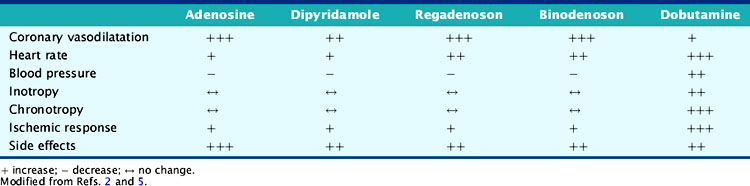
Pharmacologic stress is used in approximately 40% of the stress myocardial perfusion imaging (MPI) studies performed for the detection of coronary artery disease (CAD) in the United States.1 Approximately 75% of inpatients, 40% of outpatients, 30% of patients younger than 75 years, and 50% of patients older than 75 years cannot perform maximal exercise.2 Submaximal exercise lowers the sensitivity of exercise MPI and underestimates the degree and extent of ischemia.3 Exercise is the preferred stress modality for patients who can exercise and achieve adequate exercise endpoints, whereas pharmacologic stress is reserved for patients who have left bundle branch block (LBBB) electronically paced rhythms, or are unable to exercise or achieve adequate exercise endpoints (Table 15-1).3–5 Pharmacologic stress agents include vasodilators such as adenosine, dipyridamole, adenosine triphosphate (ATP), or the newer selective adenosine A2A receptor agonists and inotropic and chronotropic agents such as dobutamine and arbutamine. Adenosine triphosphate and arbutamine are not used in the United States and thus will not be discussed in this chapter.
Table 15-1 Factors That Favor Pharmacologic Myocardial Perfusion Imaging
From Zoghbi G, Iskandrian AE: Pharmacologic stress testing. In Garcia EV, Iskandrian AE (eds): Nuclear Cardiac Imaging: Principles and Applications. New York: Oxford University Press, 2008, pp 293-315, with permission.
PHARMACOLOGY
Adenosine
Adenosine, a small heterocyclic compound made of a purine base and a sugar ribose, is produced endogenously by endothelial and vascular smooth muscle cells in small amounts during normal cellular conditions and in larger amounts under ischemic conditions.6 The intracellular adenosine production involves two different pathways. In the S-adenosyl pathway, S-adenosyl methionine is converted to adenosine and homocysteine via the intermediary of S-adenosyl homocysteine.1,3,6 In the ATP pathway that predominates during episodes of ischemia, ATP is dephosphorylated sequentially to adenosine diphosphate (ADP), adenosine monophosphate (AMP), and finally to adenosine and phosphate by the catalytic action of a 5′nucleotidase.1,3,6 A carrier-mediated transporter transports the produced adenosine into the extracellular space, where it interacts with its cell membrane receptors on endothelial and smooth muscle cells.1,3,6 It subsequently reenters the intracellular space of endothelial cells, smooth muscle cells, or red blood cells via facilitated transport, where it gets degraded to xanthine and uric acid in a pathway that involves adenosine deaminase, nucleoside phosphorylase, and xanthine oxidase or to AMP via an adenosine kinase pathway.1,3,6 Adenosine can also be produced extracellularly from the dephosphorylation of ATP and ADP that are released from mast cells, nerve endings, or platelets.1
Adenosine receptors are divided into at least four types: A1, A2A, A2B, and A3.1,3,6,7 The different adenosine receptor subtypes, their location, and action are listed in Table 15-2.1,7,8 Activation of the A2A receptor causes coronary vasodilatation, and activation of the other receptors is responsible for undesirable effects.8 Adenosine binding to the A2 receptor activates adenylate cyclase and increases intracellular cyclic AMP production via Gs protein activation.8 This causes opening of potassium channels, inhibition of voltage-gated calcium channels, and hyperpolarization of smooth muscle cells, with resultant decrease in calcium uptake and intracellular calcium release that lead to smooth muscle relaxation and arteriolar dilation.3,8 Adenosine binding to A1 receptors inhibits norepinephrine release, stimulates endothelial-derived relaxing factor production, and activates Gi proteins that inhibit adenylate cyclase, decrease intracellular cyclic AMP, and increase potassium channel conductance—all of which result in smooth muscle contraction.1,3,8 Adenosine also has direct effects on sympathetic nerve endings, resulting in the increase in heart rate (HR) and the rise in blood pressure (BP) seen occasionally.1 Xanthine-containing compounds, such as theophylline and caffeine, competitively inhibit the action of adenosine on these receptors.4
Table 15-2 Adenosine Receptor Types, Location, and Action
| Receptor Type | Location | Action |
|---|---|---|
| A1 | ||
| A2A | ||
| A2B | ||
| A3 |
From Zoghbi G, Iskandrian AE: Pharmacologic stress testing. In Garcia EV, Iskandrian AE (eds): Nuclear Cardiac Imaging: Principles and Applications. New York: Oxford University Press, 2008, pp 293-315, with permission.
Adenosine has a very short half-life of less than 2 seconds and a rapid onset of action.4,9 Its peak hyperemic effect is reached within 2 minutes after beginning its infusion and returns to baseline within 2 minutes after termination of its infusion.4,9
Dipyridamole
Dipyridamole, a pyrimidine base, increases interstitial adenosine level by inhibiting adenosine-facilitated reuptake across vascular, endothelial, and red blood cell membranes that are responsible for its degradation.3,4 Thus, dipyridamole indirectly increases endogenous adenosine production at its receptor sites, mediating its various effects.3,4 Its peak vasodilatory effect occurs within 3 to 7 minutes from beginning of infusion, and its half-life lasts around 30 to 45 minutes.3,4
Dobutamine
Dobutamine is a synthetic sympathomimetic amine that has a direct weak β2 and α1 receptor agonist activity and a strong β1 agonist activity with dose-dependent inotropic and chronotropic effects.3,10 Dobutamine doses less than 10 μg/kg/min cause cardiac β1 and peripheral α1 receptor stimulation resulting in augmentation of stroke volume and cardiac output and minimal changes in HR and BP.3,10 Dobutamine doses over 10 μg/kg/min cause predominant cardiac β1 receptor stimulation resulting in significant dose-dependent increases in HR, contractility, and cardiac output, with minimal increase in systemic BP.3,10 The systemic BP occasionally decreases due to peripheral vasodilatation caused by more effects on the peripheral β2 receptor.3,10,11 Myocardial blood flow (MBF) increases, predominantly due to the increase in myocardial oxygen demand and a minimal direct vasodilatory effect on the coronary bed.3,10,11
Dobutamine’s onset of action is within 2 minutes of its infusion and reaches steady state after several minutes.3,10,11 Dobutamine has a 2-minute half-life and is metabolized in the liver through methylation and conjugation.11 The cardiovascular effects of the various pharmacologic stressors are summarized in Table 15-3.
MYOCARDIAL BLOOD FLOW
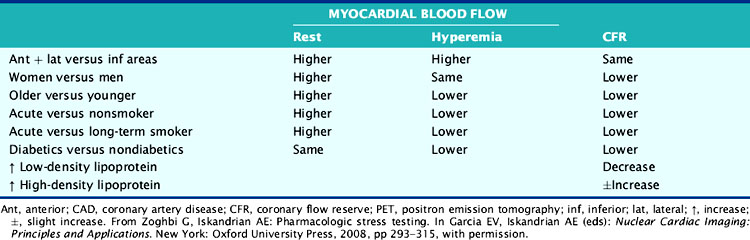
The hemodynamic significance of coronary lesions can be evaluated invasively with Doppler catheters that measure flow velocities (coronary flow reserve [CFR]), with pressure catheters that measure pressure gradients (fractional flow reserve [FFR]), or with both to measure stenosis resistance (resistance index).12,13 Coronary flow can be measured noninvasively using positron emission tomography (PET). Resting and hyperemic MBF are mainly dependent on myocardial oxygen demand expressed by the double-product of BP × HR, as well as other factors such as contractility, coronary driving pressure, left ventricular (LV) hypertrophy, blood viscosity, anemia, and microvascular disease (Table 15-4).5,14–17
Myocardial Blood Flow in Normal Patients
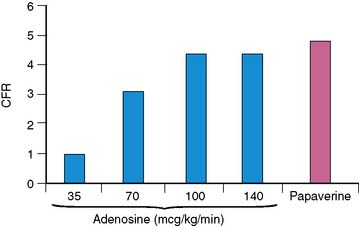
Wilson and associates first compared the effect of intravenous adenosine to intracoronary papaverine using a Doppler wire.18 In patients with normal CFR by papaverine, intravenous adenosine caused a dose-dependent increase in CFR up to a dose of 140 μg/kg/min.18 Most patients (92%) achieved near-maximal hyperemia comparable to papaverine. This compares to 50% of patients who achieved maximal vasodilatation with 0.56 mg/kg of dipyridamole infused over 4 minutes and to 84% of the subjects who received adenosine doses of 70 μg/kg/min.18 CFR was submaximal with adenosine doses of 35 and 70 μg/kg/min compared to papaverine and the 100 and 140 μg/kg/min adenosine doses (Fig. 15-1). The maximum CFR achieved with adenosine (3.4 ± 1.2) or with dipyridamole (3.1 ± 1.2) was lower than that achieved with papaverine (3.9 ± 1.1).19 The coronary vascular resistance by adenosine and papaverine was significantly lower than by dipyridamole.19 The peak flow velocity was achieved quicker with adenosine (within 55 ± 34 seconds) compared to dipyridamole (within 287 ± 101 seconds).19 In a PET study, the CFR was 4.0 ± 1.3 with dipyridamole and 4.3 ± 1.6 with adenosine, with considerable interindividual variation (range of 1.5 to 5.8 with dipyridamole; 2.0 to 8.4 with adenosine).20 The peak MBF was similar between a standard dipyridamole dose of 0.56 mg/kg (2.13 ± 0.28 mL/min/g) compared to a higher dipyridamole dose of 0.8 mg/kg (2.08 ± 0.20 mL/min/g).21
The effect of dobutamine on MBF was studied in the normal coronary arteries of 15 patients with CAD, using 13N-ammonia PET.22 The CFR increased 2.4-fold in the normal coronary arteries, corresponding to a 2.2-fold increase in the double-product.22 Dobutamine produced a lower peak MBF (2.16 ± 0.99 mL/min/g) than adenosine (3.10 ± 0.90 mL/min/g).23 Dobutamine-atropine infusion caused a greater increase in peak MBF (5.89 ± 1.58 mL/mg/min) than dipyridamole (4.33 ± 1.23 mL/mg/min), though with no difference in coronary vascular resistance.24 The 8.8-fold increase in peak MBF with dobutamine-atropine was out of proportion to the 4-fold increase in the double-product, possibly because of the atropine-mediated increase in HR.
Myocardial Blood Flow with Modified Pharmacologic Stress Protocols
The effect of 140 μg/kg/min intravenous adenosine was studied alone or in combination with supine bicycle exercise in 11 healthy volunteers using 13N-ammonia PET.25 Compared to adenosine stress alone, adenosine combined with exercise resulted in significantly higher systolic and mean BP, HR, and double-product. Adenosine combined with exercise resulted in significantly lower peak MBF (2.2 ± 0.4 mL/min/g) compared to adenosine alone (2.6 ± 0.4 mL/min/g).25 The CFR and coronary vascular resistance were also lower with the combined protocol.25 Similarly, the addition of isometric handgrip to dipyridamole caused a significant decrease rather than an increase in MBF.21
In summary, adenosine and dipyridamole cause a threefold to fivefold increase in MBF in normal coronary arteries, independent of myocardial oxygen demand (unlike dobutamine and exercise). Adenosine and dipyridamole cause a higher CFR than exercise and dobutamine.22 The hyperemic response is more predictable with adenosine than with dipyridamole and is much shorter lived.26 Addition of exercise to either adenosine or dipyridamole decreases the peak MBF compared to either alone.25
Myocardial Blood Flow in Patients with CAD
The landmark study of Gould showed that the resting MBF in dogs remained normal up to a diameter stenosis of 90%, while the CFR progressively decreased starting at 45% to 50% diameter stenosis (≈75% area stenosis).27 These results were later confirmed using PET in patients with single-vessel CAD and normal LV function.28 The resting MBF was not affected by stenosis severity, but the hyperemic MBF after intravenous adenosine progressively decreased with increasing percent diameter stenosis and decreasing minimal luminal diameter.28 The CFR started to decline at 40% diameter stenosis (≈60% area stenosis) and reached 1 at 80% diameter stenosis (≈90% area stenosis).28 Wilson et al. studied 50 patients with limited and discrete one- or two-vessel CAD using papaverine with Doppler and pressure wires.29 The CFR significantly correlated with percent area stenosis, minimal cross-sectional area, and translesional pressure gradient.29 Lesions with less than 70% area stenosis (<50% diameter stenosis) or more than 2.5 mm2 cross-sectional area had a CFR greater than 3.29
Dobutamine-induced CFR was significantly lower in regions supplied by vessels with more than 50% diameter stenoses compared to less than 50% (1.7 versus 2.3).30 A maximally tolerated dobutamine dose was compared to a standard dose of adenosine in 13 patients with CAD using PET.23 In normal segments, dobutamine caused a peak MBF of 2.16 ± 0.99 mL/min/g, which was 25% less than that achieved with adenosine (P < 0.001). In abnormal segments, dobutamine caused an MBF of 0.83 ± 0.43 mL/min/g compared to 0.90 ± 0.49 mL/min/g with adenosine (P = NS). The hyperemic response to dobutamine was in excess of that expected by the double-product and was attributed to the inotropic, oxygen-wasting, and β2 agonist effects of dobutamine. The effects of dobutamine and adenosine on MBF and CFR were compared in patients with 50% to 75% and greater than 75% diameter stenosis severity using PET.31 CFR was significantly greater with adenosine than with dobutamine stress in control subjects and remote territories not subtended by significant CAD.31 Flow heterogeneity was achieved across all coronary stenoses greater than 50% with adenosine but only in the presence of greater than 75% coronary stenoses with dobutamine.31
MYOCARDIAL BLOOD FLOW AND PERFUSION IMAGING
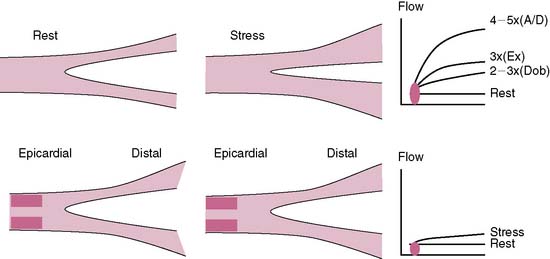
The conduit epicardial coronary arteries provide little resistance to flow under physiologic conditions.27 The MBF is primarily autoregulated by the arteriolar bed.27 In the presence of a stenosis in the epicardial artery, the translesional pressure drops, and the arteriolar bed progressively dilates proportional to the stenosis severity, maintaining normal flow to the myocardium.29 However, the arteriolar autoregulation and the vasodilatory reserve reach a maximum when the diameter stenosis approaches 85% to 90%, after which the coronary perfusion pressure drops to less than 45 mm Hg, along with a drop in the resting MBF.29 The ability of a stenosed artery to further augment the MBF during hyperemic conditions becomes limited because of the limited vasodilatory reserve of the arteriolar bed. The capillaries are important for exchange at the cellular level and contain 90% of the blood volume in the myocardium.32 A constant capillary pressure of 30 mm Hg is required to maintain hemostasis.32 During hyperemia of a stenosed vessel, the capillary resistance increases (derecruitment) to maintain a constant hydrostatic pressure in the face of dropping perfusion pressure and limited vasodilatory reserve.32 The hyperemic capillary resistance is directly proportional to the stenosis severity.32 The radiotracer uptake is dependent on its concentration, the MBF, and the capillary surface area for exchange.32 Thus the capillary surface area becomes an important limiting factor to radiotracer uptake. The more the derecruitment, the less the surface area available for radiotracer extraction, leading to a perfusion defect in the area supplied by a stenosed artery.32
Perfusion defects during stress MPI are generated from the disparity in MBF (or myocardial blood volume) and the differential radiotracer uptake between regions supplied by diseased compared to normal coronary arteries (Fig. 15-2).5 The regional tracer concentration also depends on the roll-off phenomenon in extraction at high rates of MBF.33 The first-pass extraction fraction of most radiotracers decreases when the flow rate reaches 2.5× the baseline value and leads to underestimation of the flow in relation to the myocardial tracer concentration.33 This roll-off effect is thought to be related to a disproportional increase in blood flow velocity compared to the increase in myocardial oxygen demand and may limit the ability of vasodilator MPI to detect mild to moderate coronary artery stenoses.5,33 The effect of the roll-off phenomenon is greater with technetium-99m tracers than thallium-201.33
The comparison of the results of Doppler flow wires to MPI has provided further insight into the generation of perfusion defects. Using dual Doppler flow wires to measure dipyridamole-induced relative CFR in normal (2.6) and diseased (1.1) coronary arteries, Voudris et al. showed a strong correlation (r = 0.90, P < 0.001) between CFR and the relative tracer uptake ratio on single-photon emission tomography (SPECT).34 In another study, a CFR less than 1.8 predicted the presence of a reversible perfusion defect on MPI with a 96% concordance rate.35 Other theories have been postulated as mechanisms for perfusion abnormalities during vasodilator stress such as decrease in distal perfusion pressure due to a decrease in BP, stenosis collapse, or coronary steal.36
PERFUSION IMAGING PROTOCOLS
Patients should be instructed to fast for 4 to 6 hours before the test to minimize nausea and vomiting.3,4 The methylxanthines competitively inhibit the adenosine receptors and may cause false-negative results.4 Current imaging guidelines recommend holding caffeinated products for 12 hours and dipyridamole, dipyridamole-containing medications (Aggrenox), or aminophylline for 24 hours prior to the test.3,4 Pentoxifylline can be continued prior to adenosine, and oral dipyridamole can be continued prior to intravenous dipyridamole.3,4 Some recommend holding antianginal medications for 24 to 48 hours prior to dobutamine and even vasodilator stress due to the ameliorating effect of these medications on perfusion defects.3,37 An intravenous line with a dual-port Y-connector for injecting the radiopharmaceutical and an infusion pump are needed for adenosine and dobutamine, whereas dipyridamole infusion does not require an infusion pump.3,4 Continuous electrocardiographic (ECG) monitoring and BP recordings at 1-minute intervals are recommended.4 The contraindications and endpoints of pharmacologic MPI are listed in Tables 15-5 and 15-6.
Table 15-5 Contraindications to Pharmacologic Stress Testing
| Contraindications to Dipyridamole or Adenosine |
| Contraindications to Dobutamine |
AV, atrioventricular; MI, myocardial infarction.
Reprinted from Zoghbi G, Iskandrian AE: Coronary artery disease detection: Pharmacologic stress. Zaret BL, Beller GA, eds. Clinical Nuclear Cardiology: State of the Art and Future Directions, 3rd ed. Philiadelphia: Elsevier Mosby, 2005, pp 233–253 with permission from Elsevier.
Table 15-6 Endpoints in Pharmacologic Stress Testing
| With Dipyridamole or Adenosine |
| With Dobutamine |
Reprinted from Zoghbi G, Iskandrian AE: Coronary artery disease detection: Pharmacologic stress. Zaret BL, Beller GA, eds. Clinical Nuclear Cardiology: State of the Art and Future Directions, 3rd ed. Philiadelphia: Elsevier Mosby, 2005, pp 233–253 with permission from Elsevier.
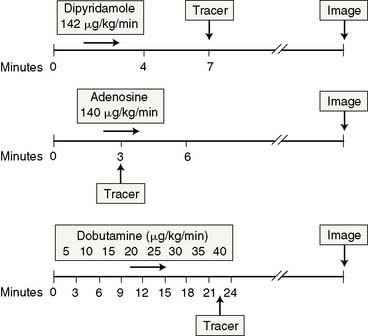
The acquisition protocols with the different stressors and radiotracers used (thallium, sestamibi, and tetrofosmin) are shown in Figure 15-3. The protocols used with technetium-labeled agents can be same-day stress/rest or rest/stress, 2-day stress/rest, or dual isotope.3,4,38,39 The thallium protocol is stress/4-hour redistribution/reinjection. The images are acquired 10 minutes after thallium injection and 40 to 60 minutes after sestamibi or tetrofosmin injection.3,4,38
Adenosine is marketed as Adenoscan and is infused intravenously at a rate of 140 μg/kg/min over 5 to 6 minutes.4,37,38 A 3- or 4-minute infusion is used in some laboratories.4,37,38 The radiotracer is injected during the third minute of the infusion.4 A modified adenosine protocol can be used in higher-risk patients such as those with history of hyperactive airway disease, liver cirrhosis with massive ascites, recent ischemic events, or low systolic BP.3,4,37,38 The modified protocol starts at a rate of 50 μg/kg/min for 1 minute followed by 75, 100, and 140 μg/kg/min at 1-minute intervals if tolerated.3,4,37,38 Low-level upright treadmill exercise at 1.7 mph and 0% grade during adenosine infusion can be used in patients without LBBB or a permanent pacemaker.3,4,37,38
Dipyridamole is injected intravenously at a dose of 0.56 mg/kg over a 4-minute period.3,4,37,38 The radioactive tracer is injected 3 to 5 minutes after termination of the dipyridamole infusion.3,4,37,38 Low-level upright treadmill exercise at 1.7 mph and 0% grade for 4 to 6 minutes shortly after completion of the dipyridamole infusion can be used in patients without a LBBB or permanent pacemaker.3,4,37,38 The radiotracer is injected during the low-level exercise, which should be continued for 2 minutes after radiotracer injection.3,4,37,38 A 50% higher dose of dipyridamole is sometimes used in Europe, but it is not certain whether the higher dose produces more coronary hyperemia.21
Dobutamine is administered intravenously at an initial dose of 5 to 10 μg/kg/min and is augmented by 10 μg/kg/min every 3 minutes until a maximum dose of 40 μg/kg/min is reached, target HR is achieved, or symptoms develop.4,37,38 Atropine in 0.5- to 1-mg increments (up to 2 mg total) is administered intravenously if the peak HR is not reached with a maximal dobutamine dose.3,40 Atropine is contraindicated in patients with myasthenia gravis, obstructive gastrointestinal tract, narrow-angle glaucoma, or uropathy.3,40 The radioactive tracer is administered at peak HR, and the dobutamine infusion is continued for an additional 1 minute (see Fig. 15-3).40
SAFETY AND SIDE EFFECTS
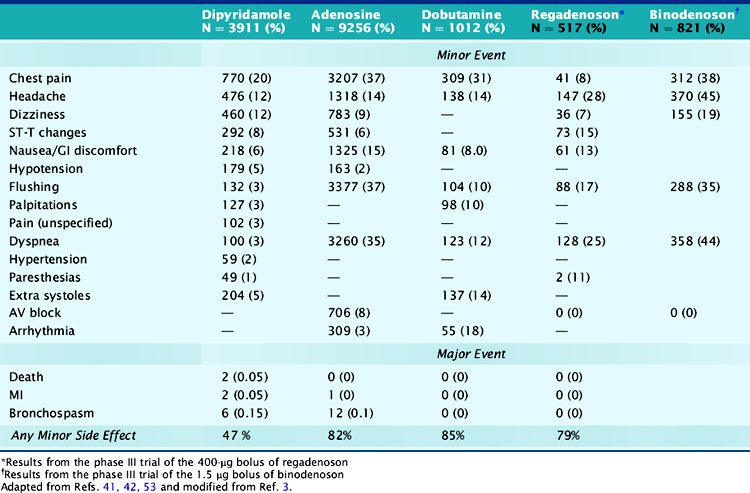
Minor side effects occur more commonly with adenosine than with dipyridamole; however, they are short lived and better tolerated and rarely require reversal with theophylline.4 Around 80% of patients developed minor symptoms during adenosine infusion and 50% during dipyridamole infusion.4,6,41,42 Clinically significant side effects such as severe chest pain, hypotension, and bronchospasm occurred in 1.6% of patients receiving adenosine.4,6,41,42 The most common side effects of adenosine and dipyridamole are listed in Table 15-7. Adenosine was better tolerated in men than women (except for flushing) and in older patients than younger patients, and was preferred over dipyridamole in the patients who received both stressors on separate days.43,44 Reversal of side effects with theophylline was required in 12% of patients receiving dipyridamole but in fewer than 1% of patients receiving adenosine.4 Chest pains during vasodilator stress are due to stimulation of the adenosine A1 receptor and thus could occur in patients with normal coronary arteries.7,45 Dyspnea during vasodilator stress is rarely due to bronchospasm or pulmonary edema and more commonly due to carotid chemoreceptor stimulation that increases the depth and rate of respiration (tachypnea).46 Adenosine and dipyridamole are well tolerated in smokers and in patients with compensated nonreactive airway disease.38,41,46 To prevent bronchospasm during stress testing, prophylactic administration of inhaled β agonists before adenosine or dipyridamole infusions has been used in patients with moderate to severe obstructive lung disease.47
Atrioventricular (AV) blocks occur in 2% of patients undergoing dipyridamole stress and in 7.6% of patients undergoing adenosine stress and are usually intermittent, transient, well tolerated, may resolve even if the adenosine infusion is continued, and are not reasons to terminate the infusion.3,37 Second- and third-degree AV blocks occurred in 4% and less than 1% of patients undergoing adenosine stress.48 In patients with first-degree AV block at baseline undergoing adenosine stress, second- and third-degree AV blocks occurred transiently in 37% and 14%, respectively, did not require any treatment, and usually resolved with decreasing or discontinuing the adenosine infusion.37,48 Concomitant use of AV blocking agents with adenosine or increasing age did not influence the incidence of AV blocks.48,49 AV block during adenosine infusion usually occurs in the first 2 to 3 minutes of the infusion, follows the hyperemic response, and does not require termination of the infusion in over 95% of cases.3,37 The radiotracer can be injected when the AV block occurs while the adenosine dose is subsequently down-titrated.3 Adenosine testing has been shown to be safe in elderly patients and in patients with significant aortic stenosis.49,50
Severe side effects with vasodilator stress agents occur rarely. In two large multicenter safety trials, the respective incidences of death and nonfatal myocardial infarction (MI) were 0 and 1/10,000 with adenosine and 1/10000 and 1.8/10,000 with dipyridamole.41,51 Severe side effects, especially bronchospasm (incidence of 8/10000 with adenosine), can be promptly reversed with 50 to 100 mg of intravenous theophylline injected over 1 minute and repeated to a total dose of 250 to 300 mg.3,37 Theophylline competitively inhibits the adenosine receptor, and its injection should be delayed for 1 to 2 minutes after tracer injection, if possible, to ensure adequate tracer uptake.3 An exaggerated hypotensive response with adenosine stress may occur in patients with severe liver disease who are being evaluated for transplant surgery. The hypotension is likely due to overdosing, as the dry weight in these patients may be only 50% of their total weight because they often have massive ascites. A titration adenosine protocol is more prudent in these patients.
Side effects occur in around 75% of patients undergoing dobutamine stress.52,53 The most common side effects are chest pains, palpitations, flushing, dyspnea, and arrhythmias (see Table 15-7).54 Chest pain during dobutamine stress testing is not a predictor of ischemia as defined by reversible perfusion defects on SPECT imaging.55 Premature ventricular complexes and nonsustained ventricular tachycardia occurred in 12% and 4.2% of patients who received dobutamine and in 31% and 6.3% of patients who received dobutamine-atropine.10,53 Ventricular arrhythmias were more frequent in patients with LV dysfunction, fixed perfusion defects, resting wall-motion abnormalities, and history of prior ventricular arrhythmias.10,11,40,53,54 In a dobutamine safety study that involved 1012 patients, there were no deaths, MIs, or malignant arrhythmias.53 The side effects of dobutamine can be reversed by a short-acting β-blocker such as esmolol.4 We believe that dobutamine should not be used shortly after acute MI and possibly also in patients with large aortic abdominal aneurysm and those with atrial fibrillation or history of serious ventricular arrhythmias.
HEMODYNAMIC EFFECTS
Vasodilators
Adenosine and dipyridamole cause modest decreases in systolic, diastolic, and mean BP and a modest increase in HR, with a greater effect observed with adenosine than dipyridamole.38 Dipyridamole increased the HR by 11 ± 7 beats/min, decreased the mean BP by 10 ± 3 mm Hg, and increased the cardiac output by 34%.56 Adenosine increased the HR by 14 to 17 beats/min and decreased the systolic BP by 10 to 18 mm Hg and the diastolic BP by 8-9 mm Hg.41 In patients undergoing adenosine stress, the HR increased in 94% of patients, the systolic BP decreased in 85% of patients, and the diastolic BP decreased in 80% of patients.6 The HR response to adenosine infusion was diminished in patients with diabetes mellitus (DM) and normal perfusion on SPECT imaging, most likely due to diabetes induced cardiovascular autonomic neuropathy.57 The systolic BP decreased transiently to less than 80 mm Hg in 2.5% of patients and increased paradoxically in 13% of patients.6 The pulmonary capillary wedge pressure slightly increased in normal subjects and more so in patients with CAD.41 The decrease in BP is due to a drop in the systemic vascular resistance. The increase in the pulmonary capillary wedge pressure is due to an increase in venous return and preload, increased diastolic stiffness, and coronary turgor in normal patients, and also due to ischemia-induced diastolic and systolic LV dysfunction in patients with CAD.36 The increase in cardiac output is primarily due to an increase in HR.36 Adenosine and dipyridamole produce their coronary vasodilatory effects independent from their peripheral hemodynamic effects. Thus, CAD detection accuracy is independent of the vasodilator peripheral hemodynamic changes.58,59
Dobutamine
The hemodynamic effects of dobutamine are comparable to those observed with submaximal exercise.3,10 A 40 μg/kg/min dobutamine dose increased the systolic BP by 27 mm Hg and the HR by 45 beats/min and decreased the diastolic BP by 17 mm Hg.3,60 Incremental dobutamine doses progressively increased the HR while the BP increased and leveled at a dose of 20 μg/kg/min.3,60 Hypotension during dobutamine stress, defined as ≥ 20 mm Hg drop in BP from baseline, occurred in 14% to 20% of patients and is due to peripheral vasodilatation, not to ischemia.3,11 As such it is not predictive of the presence or extent of wall-motion abnormality or LV dysfunction and has no prognostic implications as seen with exercise-induced hypotension.3,11 The hypotensive response occurs more commonly in patients with advanced age, high baseline systolic BP, dynamic LV outflow obstruction, and small hyperdynamic LV.3,10,11 In one study, however, dobutamine-induced hypotension correlated with the number of ischemic segments on perfusion imaging in patients with previous MI.10 Dobutamine-related sinus node deceleration is defined as an initial increase followed by a decrease in HR and occurs in 7% to 19% of patients.3,10,11 It results from activation of cardioinhibitory receptors that activate the Bezold-Jarisch reflex. 10,11
ISCHEMIC RESPONSE
Vasodilators
Adenosine or dipyridamole can cause ischemia by producing coronary steal that could be collateral dependent or transmural. Collateral-dependent steal results from decrease in collateral flow distal to a stenosed artery because of differential flow decrease in the donor vessel.61,62 Transmural steal is less common and less severe and occurs from the subendocardial to subepicardial region, owing to the difference in residual vasodilatory reserve in the subendocardium and subepicardium.61–63 Systemic hypotension that occurs due to systemic vasodilatation exacerbates the steal phenomenon.62 Markers of ischemia are ST-segment depressions, typical angina pectoris, and regional wall-motion abnormalities.61 Most perfusion defects, however, are not due to ischemia but rather to disparity in regional blood flow as a result of variations in hemodynamic severity of coronary stenoses.
Ischemic ST-segment changes due to vasodilator stress are usually depression and rarely elevation. Ischemic changes occurred in 15% to 40% of patients with CAD undergoing dipyridamole stress and in less than 10% of an unselected population.64,65 ST depressions occurred in 7.6% of 959 patients who underwent adenosine stress and were more common in women (64%) compared to men (36%).66 Adenosine-induced ischemic ST-segment changes have been correlated with higher baseline and peak systolic BP, greater increase in BP and HR, occurrence of typical angina during adenosine infusion, extensive CAD, presence of collaterals on coronary angiography, extensive and severe perfusion abnormalities, and transient ischemic dilation (TID) (Fig. 15-4).66–68 ST depressions occur less commonly than perfusion defects but have a specificity of 90% for CAD detection.68 Adenosine-induced ischemic ECG changes with normal perfusion on MPI occurred in 1% to 2% of patients, of whom 80% to 88% were women.69–71 A high event rate was reported in these patients in two studies but was not supported by another study from our own group.69–71