Physician
Year
Technique
Reference
Francois-Franck
1899
Sympathectomy
Alexis Carrel
1910
Vascular anastomosis
[3]
Claude Beck
1932–1945
Pericardial abrasion
Pectoral muscle graft
Arterialization of the coronary sinus
Vineberg
1945
LIMA placed into LV myocardium
[6]
Bailey and Lemon
1957
Endarterectomy
[7]
Sones
1958
Selective coronary angiography
Judkins
1967
Kolessov
1964
LIMA bypass
[11]
Sen
1965
Myocardial acupuncture
[12]
Sabiston
1962
Aortocoronary bypass with reversed saphenous vein
DeBakey
1966
Favaloro
1968
Coronary bypass of multivessel disease
Multiple
1976
Nonrandomized studies suggest survival advantage from revascularization
Before the development of selective coronary arteriography, the diagnosis of coronary atherosclerosis was made on clinical grounds and confirmed objectively through electrocardiography and exercise testing. Symptoms or signs prompting a diagnosis of CAD were associated with a survival expectation of about 10 years [22]. There were few methods to improve prognosis estimation or to alter this natural history, and the obstacles to be overcome in improving myocardial perfusion in an individual patient were poorly understood.
In 1958, Mason Sones performed the first selective coronary angiogram. This technique improved understanding of the natural history of coronary atherosclerosis and lit the way for successful revascularization of the heart [9, 10]. Observational data from numerous patients undergoing coronary angiography revealed that the clinical diagnosis of CAD, based on the patient’s history, physical examination, and electrocardiogram (ECG), does not invariably indicate severe coronary obstruction. Conversely, severe coronary obstruction may be present with little outward manifestation [23–27]. Describing disease severity required not only a measure of the degree of luminal narrowing in order to determine the likelihood of blood flow limitation but also some measure of its extent. The simplest measure of extent is the sum of the number of major vessel regions (left anterior descending [LAD], circumflex, right coronary artery, left main coronary artery [LMCA]) with severe stenosis. Even this very crude measure, when used in combination with an estimate of left ventricular (LV) systolic function (Fig. 26.1), could determine whether a patient had a high, intermediate, or low risk of dying. According to Sones and coworkers’ data, 5-year cardiac mortality ranged from 7 % in patients with disease in only 1 vessel and normal ventricular function to 100 % in those with LMCA stenosis and severe contractile dysfunction [23, 25].
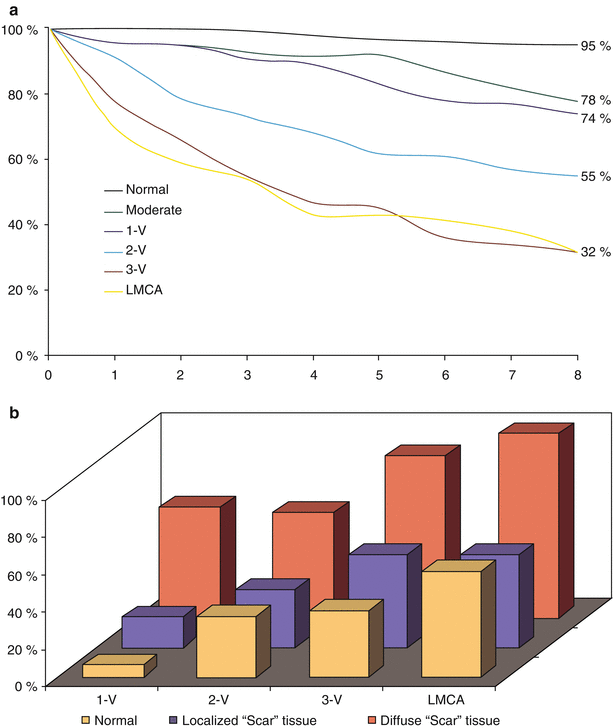

Fig. 26.1
Survival according to number of major vessels diseased. (a) In several studies of the relationship between coronary angiographic findings and long-term outcome, Brushke, Proudfit, and Sones used a simple classification scheme of one, two, three vessels (V) or left main coronary artery (LMCA) with severe stenosis [23–27]. Comparative survival analysis reveals that any evidence of atherosclerosis alters prognosis, but the extent of severe stenosis is a powerful predictor of survival. (b) In addition to the number of major vessels with severe stenosis, the severity of left ventricular dysfunction proved to be a powerful predictor of 5-year survival (From Bruschke et al. [25]. Reprinted with permission from Wolters Kluwer Health)
The accuracy of an angiographic diagnosis and the increased risk of MI in patients with the diagnosis of CAD led to supposition that disease severity or the characteristics of coronary stenoses would be useful to identify patients or vessels with a high risk for MI. In those patients, timely application of revascularization may prevent the event. This concept arose from assumptions that the number of severely stenotic vessels reflects total disease burden and that larger, more complex lesions (from the angiographer’s standpoint) are more prone to progression than smaller, less complex ones are. Both assumptions have support from observational studies [28–34]. In medically treated patients, single-, double-, or triple-vessel disease is associated with a yearly risk of fatal or nonfatal events of 1.2, 3.1, and 4.7 %, respectively [30–32]. However, predictive accuracy is poor, and MI is often the result of disease progression where severe stenosis was not previously present. Therefore, beyond establishing the diagnosis of coronary atherosclerosis, angiography cannot be used to predict incident MI or its subsequent location in an otherwise stable individual. This principle also applies to the examination of the impact of CAB surgery on survival.
Angiography effectively identifies who is most likely to die of MI, rather than who is most likely to have one. The central principle of all methods of angiographic disease classification is that the combination of LV systolic function and the amount of potentially ischemic myocardium determine survival prognosis [23, 25, 27, 35–43]. Normal LV function identifies a population whose next “event” is likely to be nonfatal MI, whereas LMCA or multivessel disease with severe LV dysfunction identifies a patient population whose next “event” will probably be death [44].
An important advance in this simple yet powerful classification scheme addresses the complexity of individual coronary lesions with respect to the difficulty of their treatment. Sones’ simple classification identified patients in need of urgent treatment when surgery was the only option. A recent augmentation of this classification scheme, the SYNTAX score, incorporates Sones’ method with information describing each individual lesion, aiding not only the decision to treat but indicating which method, percutaneous or surgical, will be more effective (Fig. 26.2, Table 26.2) (http://syntaxscore.com) [45, 46].
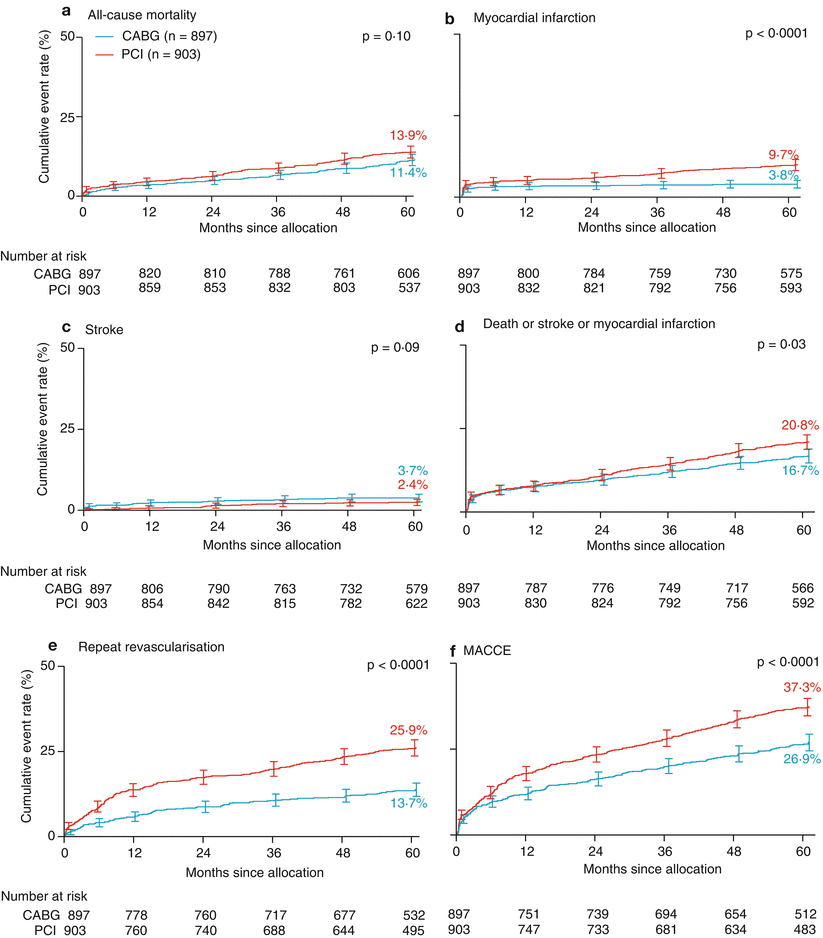

Fig. 26.2
Outcomes in the SYNTAX trial according to score category and extent of disease. Patients’ comparative outcomes after revascularization were examined categorically to establish a “dose-response” relationship between the SYNTAX score and outcome, providing rough guidelines to assist with the choice of revascularization method. Shown are the 5-year cumulative major adverse cardiovascular event rates (computed by the Kaplan–Meier method) for the percutaneous coronary intervention (PCI) and the coronary-artery bypass grafting (CABG) groups in the full cohort (column A), patients with left main coronary artery disease (column B), and patients with three-vessel disease (column C). Groups were divided according to their scores into categories of disease complexity: low (0–22), intermediate (23–32), and high (≥33). For each increase in scoring group, the complication and failure rate rose for patients in the PCI group but not in the CABG group. Thus, patients with intermediate and high SYNTAX scores had a clear benefit from surgical therapy, whereas patients with a low score are equally well treated with both methods (From Mohr et al. [45]. Reprinted with permission from Elsevier Limited)
Table 26.2
Factors used to compute the SYNTAX score
The following characteristics are used to score each coronary lesion with a diameter stenosis of ≥50 % in a vessel at least 1.5 mm long: |
Dominance (right or left) |
Total occlusion |
Trifurcation |
Bifurcation |
Aorto-ostial |
Severe tortuosity |
Length >20 mm |
Heavy calcification |
Thrombus |
Diffuse disease |
Coronary Artery Bypass Graft Surgery
Natural History of a Saphenous Vein Graft
In its first iteration, CAB surgery exploited the redundancy of the human venous system to find a source for conduit autotransplantation, the saphenous vein. This conduit and its response to “arterialization” remain among the principal limitations of treatment durability. The life of a saphenous vein graft (SVG) may be divided into three stages: a response to autotransplantation, fibrous transformation, and atherothrombotic evolution. The time course for each period is subject to individual variation. Careful handling of the SVG during surgery is required to avoid injury and thrombosis resulting in early closure after implantation [47–50]. Even when the graft is placed with meticulous care, the endothelium of the saphenous vein is quickly overwhelmed by arterial pressure and flow conditions. Platelets and fibrin coat the vessel lumen, later to be replaced by smooth muscle cells that reinforce the vessel wall by producing ground substance and short elastic fibers. Chronic medial ischemia results in a loss of muscle cells and replacement by fibrous tissue. The evolution of fibrous medial replacement may alter the shape and flow characteristics of the graft through contraction, distortion and increased rigidity. With time, the predominant appearance of the vessel wall is that of dense fibrous tissue. These early responses to saphenous vein autotransplantation combine to occlude 12–19 % of vein graft bypasses within 1 year from surgery [51, 52].
After fibrous transformation is complete, a period of relative histological and clinical stability lasts for an average of 5–6 years [53, 54]. Meanwhile, the proximal native vessel whose flow requirements are reduced suffers accelerated disease progression, often closing quietly to result in complete bypass graft dependence [52, 55]. After this “grace” period, continued accumulation of intimal connective tissue, perhaps complicated by persistent endothelial dysfunction, provides fertile ground for the development and progression of atherosclerosis, eventually leading to thrombosis and graft closure. Five years after surgery, the proportion of occluded SVGs has risen to 29 %, reaching 51 % at 12 years (Table 26.3) [51].
Table 26.3
Saphenous vein graft patency
Early | 1 year | 2.5 years | 5 years | 7.5 years | 10 years | 12.5 years | ≥15 years | |
|---|---|---|---|---|---|---|---|---|
N (Grafts) | 4,592 | 3,706 | 469 | 1,889 | 495 | 856 | 227 | 353 |
Occluded grafts | 12 % | 19 % | 29 % | 25 % | 40 % | 40 % | 51 % | 50 % |
>50 % DS | 0 % | 2 % | 4 % | 16 % | 26 % | 26 % | 28 % | 28 % |
Mild | 0 % | 3 % | 6 % | 20 % | 17 % | 20 % | 14 % | 12.5 % |
No stenosis | 88 % | 76 % | 61 % | 39 % | 17 % | 14 % | 7 % | 9.5 % |
Impact on Survival
With all of the limitations of the reversed saphenous vein conduit, CAB surgery is very effective in providing relief from angina pectoris. Observational studies similar to those reported by Sones and coworkers [23, 25] repeated in the CAB population provided the first evidence that revascularization of the epicardial coronary arteries altered the natural history of CAD (Fig. 26.3) [18–21]. After CAB surgery, the survival curves for each category of disease severity, which had been distinctly different with medical therapy alone, were almost superimposed on one another. Patients with coronary atherosclerosis of all grades of severity enjoyed a survival expectation somewhere between the previously reported values for unoperated patients with 1- and 2-vessel disease.
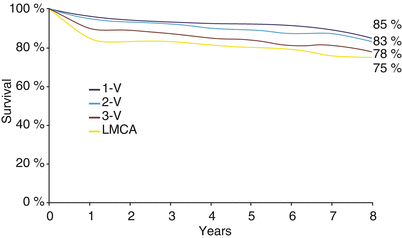

Fig. 26.3
Survival according to the number of major vessels diseased: the effect of surgical revascularization. One of several observational studies of the impact of surgical revascularization on patients with coronary disease, this study from the Texas Heart Institute repeated the natural history study of Sones and coworkers in bypassed patients. Survival over a period of 8 years is still stratified by disease severity, but survival in the most severely affected patients is markedly different from that reported by Sones and coworkers. This was among the first studies to establish a survival benefit from revascularization and suggested that angiographic risk stratification would be useful in determining which patients would realize that benefit (From Hall et al. [18]. Reprinted with permission from Texas Heart Institute)
Opinion about the value of CAB surgery at its inception was far from uniform [18, 56–71], giving rise to randomized trials of surgical therapy [72, 73]. From three large randomized trials and one registry [74–83] come the most reliable data concerning the impact of coronary revascularization on the natural history of CAD (Table 26.4). These landmark studies provided the first incontrovertible evidence to guide revascularization decisions. However, their data are frequently misquoted. The randomized trials of CAB surgery compare two philosophies of therapy for coronary occlusive disease: referral for revascularization on recognition of disease versus medical management with revascularization reserved for patients with unacceptable symptom control. They are not true comparisons between surgical and medical therapy for CAD. In each study, patients assigned to the “medical” arm were allowed to undergo CAB surgery if they or their physician deemed it necessary. This is especially important when considering that at the time period of apparent superiority of surgery over “medicine” for specific subgroups, one-third to slightly less than one-half of medically assigned patients had undergone CAB surgery.
Table 26.4
The three major surgery trials
VA cooperative study (n = 686) | CASS (n = 780) | ECSS (n = 768) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Angina | ||||||||||
Mild/moderate | 0 % | 26 % | 0 % | |||||||
Asymptomatic | 0 % | 74 % | 57 % | |||||||
% Stenosis in ≥1 lesion | ≥50 % | ≥70 % | ≥50 % | |||||||
Ejection fraction | >35 % | >30 % | >50 % | |||||||
Operative mortality | 5.8 % | 1.4 % | 3.3 % | |||||||
1 year | 18 years | 22 years | 10 years | 12 years | ||||||
Survival (%) | M | S | M | S | M | S | M | S | M | S |
Overall | 58 | 58 | 33 | 30 | 25 | 20 | 79 | 82 | 67 | 71* |
2-vessel disease | 69 | 55 | 34 | 30 | 31 a | 24 a | 83 | 88 | b | b |
3-vessel disease | 50 | 56 | 32 | 25 | – | – | 75 | 76 | 68 | 78 a |
3-vessel disease, low LVEF | 39 | 51* | 21 | 24 | 11 | 12 | 57 | 75 | – | – |
Randomized surgical trials were conducted in the 1970s when anesthetic techniques, monitoring, postoperative care, and medical therapy were less sophisticated than today. Improvements in surgical technique and postoperative care have reduced the risk of perioperative MI and death [86]. This almost certainly reduces the risk-to-benefit ratio of a patient treated today. The current CAB surgery population is higher risk than the majority of patients enrolled in these trials. Nonetheless, expected operative mortality for a first CAB operation is about 1.5 %. The operative mortality rate is of course influenced by individual patient risk factors such as the severity of LV dysfunction, number of vessels requiring bypass, age, the presence of diabetes mellitus, gender, coexistent peripheral vascular disease, renal insufficiency, and pulmonary disease. Among patients more than 70 years of age, operative mortality is increased two to three fold [87–89]. Patients more than 80 years old have an expected mortality of 8 % or more [90, 91]. Because of changes in anesthetic and surgical care surrounding CAB surgery and advances in medical therapy that address the natural history of CAD, recent trials of surgical versus medical treatment are woefully underpowered and provide little useful information [92].
The randomized trials confirmed that CAB surgery improves symptoms of angina and exercise capacity [73, 93] but found, surprisingly, that the likelihood of nonfatal Q-wave MI was not affected [94]. Despite this, the survival of specific subpopulations of patients with coronary occlusive disease was improved by early referral for surgical revascularization. Surgery improved survival for patients in whom angiography and physiological studies implied that the next MI would be fatal. These populations were well defined in subgroup analyses of the various trials (Table 26.5) [84, 95–101].
Left main stenosis >50 % |
Left main equivalent (proximal LAD + Cx or proximal 3-vessel region stenosis) |
Depressed LVEF or positive TMT with: |
3-vessel region stenosis >70 % or |
2-vessel region stenosis >70 %, including proximal LAD |
Veterans Affairs Cooperative Study
The Department of Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery was the first large-scale randomized trial of CAB surgery versus medical therapy. Between 1972 and 1974, 686 male patients with symptomatic CAD were enrolled in the study and randomly assigned to either medical or surgical therapy (Table 26.4). To qualify for the study, patients had to have stable angina for 6 months, including a 3-month trial of medical management, ECG evidence of a previous MI or ischemic changes at rest or with exercise, and at least 50 % diameter stenosis of at least 1 major coronary artery. Patients assigned to medical therapy were not prohibited from later bypass grafting if it was deemed necessary [102]. At 22 years’ follow-up, 66 % of patients assigned to the medical therapy arm had undergone CAB surgery [81].
Surgery provided more complete relief of symptoms than did medical therapy and, by two and a half years, a survival benefit was apparent in patients with LMCA stenosis [80, 100, 103]. Patients classified as high risk (i.e. those with stenosis of the 3 main vessel regions and ejection fraction <50 %) had improved survival that was maximal at about 7–11 years as a result of early surgical bypass [79, 80]. Extended follow-up revealed that the two therapeutic philosophies produced similar long-term outcomes (Fig. 26.4) [80, 81].
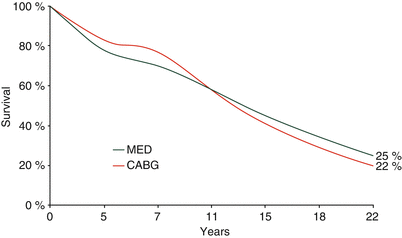

Fig. 26.4
Veterans Affairs (VA) Cooperative Surgery Trial: 22-year follow-up. The VA randomized trial established that surgical revascularization could improve the survival of patients with the most severe coronary disease. As the survival curves show, the temporary benefit of surgical revascularization gives way to an increased late hazard as saphenous vein graft atherosclerosis appears, resulting in myocardial infarction and a requirement for repeat revascularization procedures. MED medical treatment, CABG coronary artery bypass graft (From Peduzzi et al. [81]. Reprinted with permission from Elsevier)
Coronary Artery Surgery Study (CASS)
In 1974, the Coronary Artery Surgery Study (CASS) was initiated to compare the results of an initial strategy of surgical bypass combined with medical therapy versus medical therapy alone for the treatment of CAD accompanied by mild or no symptoms (Table 26.4) [84, 104]. The CASS consisted of 2 parts: a 15-center observational registry and an 11-center randomized trial. To be included in the study, patients had to have >70 % diameter stenosis of 1 or more coronary arteries. Exclusion criteria were Canadian Cardiovascular Society Class 3 or 4 angina severity, prior CAB surgery, age >65 years, LMCA stenosis >70 %, a left ventricular ejection fraction (LVEF) <35 %, overt heart failure, and shock. Of the 24,959 patients who were enrolled in the registry between July 1974 and May 1979, 16,626 were considered to be candidates for participation in the randomized trial. After baseline coronary angiography, 2,099 patients were offered the opportunity to participate; 780 agreed and were randomly assigned to either immediate surgery or medical treatment.
Analysis of 10-year follow-up data revealed that although surgery improved symptoms, there was no difference in cumulative survival (79 % medical vs. 82 % surgical) or event-free survival (69 % medical vs. 66 % surgical) in the total cohort [84]. Of the 390 patients randomly assigned to the medical treatment strategy, 6 % crossed over to surgery within 6 months; this percentage increased to 40 % by 10 years (22 % with 1-vessel disease, 42 % with 2-vessel disease, and 53 % with 3-vessel disease). However, patients with an LVEF of less than 50 % benefited from early surgical referral; their survival was 79 %, versus 61 % with medical therapy. Patients with a combination of 3-vessel disease and an LVEF <50 % saw the greatest benefit. Conversely, patients with an LVEF greater than 50 % did better when surgery was reserved for the treatment of unacceptable symptoms (event-free survival was 75 % with medical treatment vs. 68 % with surgery).
The European Coronary Surgery Study
In the European Coronary Surgery Study, 767 men under the age of 65 with mild or moderate angina and normal or minimally abnormal LV function (Table 26.4) were randomly assigned at 12 clinical centers to either medical or surgical treatment [78, 85]. To qualify for this study, patients had to have luminal diameter narrowing >50 % in at least 2 major coronary arteries and symptoms for ≥3 months. The primary endpoints were symptoms, functional status, and survival.
At 5 years, there was a significantly higher survival rate in the surgical treatment group (92.4 %, vs. 83.1 % in the medical group (83.1 %) [78, 85]. During the next 7 years of follow-up, mortality was somewhat higher in the surgical group than the medical group, although survival at 12 years still favored surgery (70.6 %, vs. 66.7 % in the medical group). Meanwhile, 36 % of the medically assigned patients had crossed over to surgery [85]. In-depth analysis of the effect of coronary artery anatomy on survival revealed a survival benefit in patients with 3-vessel disease and those with 2-vessel disease and proximal LAD stenosis. The most important observation arising from the European Coronary Surgery Study that was also made in the CASS registry was that little benefit can be expected from surgical revascularization in patients with a normal treadmill exercise test.
Meta-Analysis of Coronary Bypass Surgery Trials
This analysis combined data from 6 major randomized studies of CAB graft surgery versus medical therapy; 2,499 patients were studied [105]. Of these patients, 21 % had an abnormal LVEF (<50 %), 10 % had 1-vessel disease, 32 % had 2-vessel disease, 51 % had 3-vessel disease, and 7 % had LMCA disease. The internal mammary artery (IMA) was used for only 10 % of bypass grafts. Early referral for surgical therapy provided a 33 % reduction in 7-year mortality compared with medical therapy and a 70 % reduction in mortality for patients with LMCA stenosis. In 3-vessel disease, there was a 45 % reduction in mortality in the surgical group. In 1- and 2-vessel disease groups, there were far fewer events, and the trend favoring surgery was much weaker, although certain high-risk subsets appeared to benefit. Patients with both low and normal LVEF appeared to benefit from surgery, having reductions in mortality of about 50 and 30 %, respectively. Physiologic testing predicted a survival benefit from early surgical referral. Symptom severity did not.
Knowledge of LV function or the outcome of an exercise study is necessary to identify patients with multivessel disease who will reap a survival benefit from surgical revascularization (Fig. 26.5). Like angiography, the exercise test does not reliably predict MI in patients with known, clinically stable CAD, yet its ability to predict survival is profound. Also like angiography, both evidence of myocardial ischemia (ST-segment response or imaging correlate) and LV function are important. The exercise correlate to ventricular function is exercise capacity, which is at least as effective as, if not more effective than, ST-segment response as a predictor of mortality (Fig. 26.6) [36, 106–126]. In fact, exercise tolerance rivals angiographic risk classification in predicting the survival benefit of CAB surgery [127]. In 241 medically treated patients with angiographic evidence of significant coronary artery stenosis and ST-segment depression ≥2 mm during a Bruce protocol treadmill exercise test, those completing only stage I had an 8-year survival rate of 45 %, whereas those reaching stage IV or more had a survival rate of 93 %. Nonfatal acute events occurred in about 20–35 % of patients whatever the completed stage of the exercise test [111].
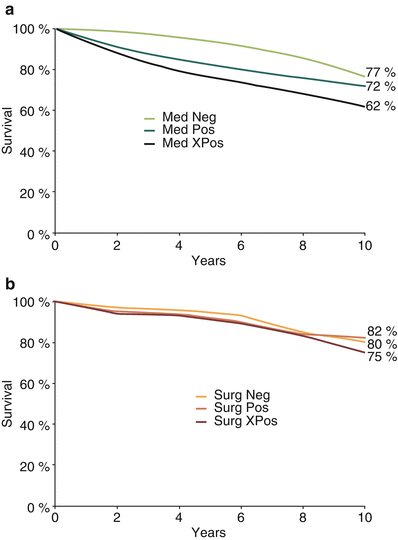
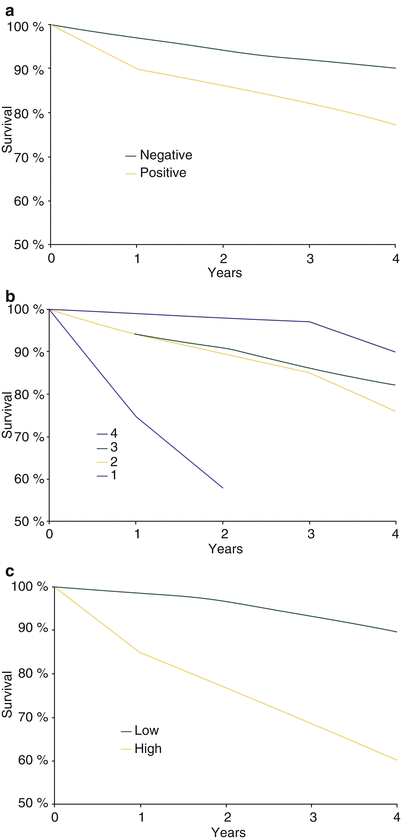

Fig. 26.5
Physiological testing and the effect of revascularization. In the European Cooperative Surgery Study, a survival benefit from coronary bypass surgery during 10 years’ follow-up could be predicted by exercise test performance. (a) A medically treated patient with a strikingly positive study (Med Pos) saw a much worse prognosis than a patient with a negative (Med Neg) or simply positive study with no high-risk criteria (Med XPos). A strikingly positive test was defined as the presence of two out of three possible measures: a maximal heart rate ≤120 bpm, a maximum workload of ≤100 W, and ST-segment depression of ≥1.5 mm. (b) After coronary bypass surgery (Surg), these differences in outcome were much smaller. The bypassed patient with a strikingly positive study enjoyed a survival prognosis similar to that of the medically treated patient with a negative study (From Varnauskas [85]. Reprinted with permission from Massachusetts Medical Society)
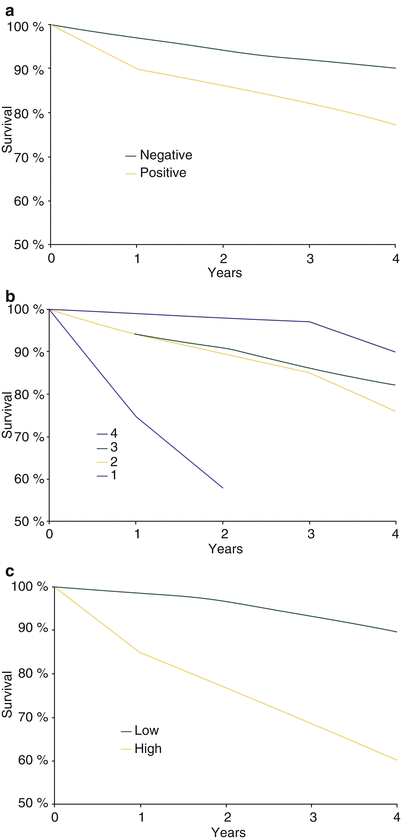
Fig. 26.6
Physiological testing and survival prognosis. (a) In patients undergoing diagnostic exercise-ECG study who are not later referred for coronary artery bypass surgery ST-segment analysis is useful to identify a population at increased risk of dying. (b) In patients with recognized coronary artery disease, exercise capacity is a more powerful measure of prognosis than ST-segment analysis. (c) When several variables are combined, such as the ST-segment response, the exercise time, maximum heart rate achieved, and the presence of angina, discriminatory power is even greater. Low risk includes patients with a negative test or exercise duration at stage 4 or higher and/or a maximum heart rate ≥160 bpm. High risk is a combination of abnormal ST-segment response and exercise time at less than stage 3 [106]
Poor exercise tolerance indicates low reserve capacity and a reduced survival prognosis. Coexistent ST-segment depression or imaging evidence of ischemia suggests that coronary artery stenosis is the cause of that limitation and that, just as with severe disease as described angiographically, the next episode of disease progression (whose occurrence is unpredictable) will probably be fatal [119]. Therefore, both ST-segment response and exercise tolerance or some measure of fitness can be used to determine whether a patient requires revascularization [128, 129].
Myocardial infarction is the most important cause of death in patients with CAD. Although one would assume that tests predicting death would also predict MI, such predictive ability has been difficult to demonstrate in clinically stable patients. One would also assume that a treatment such as CAB surgery that improves survival must reduce the risk of MI. The principal means by which surgery alters survival prognosis seems to be a reduction in infarct size and infarct-related mortality, rather than any influence on the incidence of infarction or unstable angina [130–133]. After major noncardiac operations on 1,600 CASS registry patients, operative mortality for individuals with prior CAB surgery was similar to that of patients without significant CAD (0.9 % vs. 0.5 %, p = 0.42). Patients with significant CAD but without prior CAB graft surgery had an increased operative mortality (2.4 %, p = 0.009). There was no difference in the incidence of perioperative MI between the two groups. In the same registry, there were 985 medical and 369 surgical patients who had an MI out of the hospital within 3 years after enrollment. Surgical treatment reduced the likelihood of sudden death (12 % surgical vs. 20 % medical, p = 0.001) and death after 30 days (21 % surgical vs. 36 % medical, p < 0.0001). Medically treated patients with 3-vessel disease or LV dysfunction were at highest risk of immediate death after MI. In surgically treated patients, LV dysfunction or prior MI predicted an increased risk of immediate death, but the presence of multivessel disease was rendered irrelevant by the surgical procedure [134].
Arterial Bypass Conduits
The limitation of SVG durability is evident clinically in the return of symptoms in surgically bypassed patients after 5 years and the increased morbidity that follows [51, 55, 80, 135–143]. Clinical events such as recurrent angina, repeat revascularization procedures, and mortality mirror the rate of graft occlusion. At 5 years after bypass, 2 % of patients require a second procedure and all-cause mortality is only 6 %. By 12 years, less than one-half of patients in whom only SVGs are used avoid MI or a second operation [143].
In contrast to the SVG, IMA conduits are remarkably resistant to atherosclerosis, having 10-year patency rates of 80–95 % (Fig. 26.7) [141, 144–149]. The IMA performs best when used to bypass the LAD. Low rates of disease progression and higher long-term patency translate to improved symptom control and survival [147]. Actuarial survival is improved with the use of IMAs versus SVGs alone in 1-, 2-, and 3-vessel disease (Fig. 26.8) [147, 150]. The importance of stenosis of the LAD and the success of the IMA in bypassing it has led to the almost routine use of the IMA-to-LAD bypass. Its durability is of profound importance to survival in patients with treated diabetes mellitus or end-stage renal disease necessitating hemodialysis [151, 152].
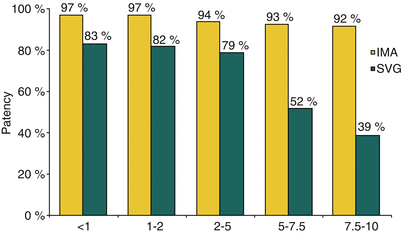
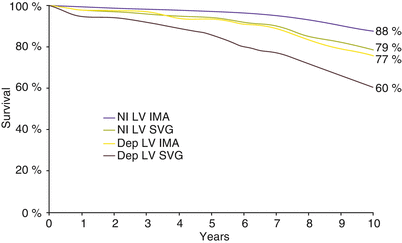
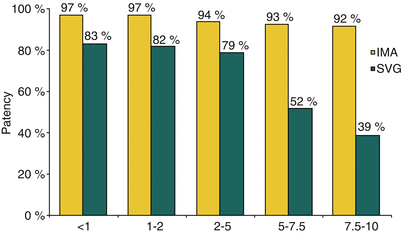
Fig. 26.7
Internal mammary artery (IMA) and saphenous vein graft (SVG) patency. With time, the saphenous vein graft will succumb to an accelerated form of atherosclerosis. The IMA is relatively resistant to this process. By 10 years, only 39 % of vein grafts remain patent, compared to 92 % of IMA grafts (From Lytle et al. [141]. Reprinted with permission from Elsevier Limited)

Fig. 26.8
Internal mammary artery and survival. The use of the internal mammary artery (IMA) as a bypass conduit to the left anterior descending coronary artery provides a profound survival benefit. This benefit was apparent for patients with a left ventricular ejection fraction (LVEF) <50 % (p < .0001) or >50 % (p = .0002). In fact, patients with an LVEF <50 % who received an IMA bypass had survival comparable to that of patients with an LVEF >50 % who received only saphenous vein grafts (From Loop et al. [147]. Reprinted with permission from Massachusetts Medical Society)
The success of the left IMA as a conduit for bypassing the LAD prompted speculation that the use of bilateral IMAs and other arteries in place of the reversed saphenous vein would greatly enhance the durability of the procedure. The use of both IMAs may be associated with an increased risk for sternal wound infection and mediastinitis. This is particularly true in patients who are female, insulin dependent, or undergoing emergency or reoperative procedures, and for patients with chronic obstructive pulmonary disease [153–159]. However, in most observational studies, using bilateral IMAs has no discernable effect on operative mortality [160]. The use of both IMAs as bypass conduits appears to confer greater graft patency and a lower risk of recurrent angina and the need for repeat revascularization. However, although several observational studies suggest that using bilateral IMAs improves long-term survival, this improvement has not yet been established [161–169].
Advances
Other arterial conduits studied as potential replacements for the SVG include the radial, gastroepiploic, and inferior epigastric arteries. Using such conduits would make it possible to perform multivessel revascularization without using an SVG. In fact, it is possible to avoid creating a proximal aortic anastomosis, instead creating a composite graft with all proximal anastomoses on the IMA. An aortic “no touch” procedure would provide both a reduced risk of perioperative stroke and arterial bypass conduits with greater longevity than the SVG. Although this approach is an excellent method for all arterial bypass because it avoids manipulation of severely diseased or calcified aortas, its safety and efficacy for general use is not established, and any difficulty with the proximal parent graft results in failure of the procedure [170–173].
Generally, non-IMA arterial conduits are less effective than IMA conduits. Early and mid-term angiographic follow-up (i.e., 1–5 years) of non-IMA arterial grafts reveals their patency to be a more a function of the target for anastomosis (i.e., right coronary artery, circumflex, LAD) and the severity of stenosis in the native vessel than of the type of conduit used [174, 175]. The radial artery graft, which is more prone to atherosclerosis than the IMAs and which must be used as a free graft, has received the most attention. Radial grafts appear to have a patency rate that is similar to or slightly better than that of SVGs [176–181], and clinical outcomes after radial artery grafting are as good or better [176, 182]. In all likelihood, the long-term performance of the left IMA (LIMA) in improving survival will make it difficult to provide irrefutable evidence that all arterial grafting is superior to the use of LIMA grafts and SVGs. The best available evidence suggests that multiarterial grafting is superior to LIMA/SVG [183]. However, this is a propensity-based analysis and cannot exclude the possibility of bias. The true test of patency and performance awaits the outcome of randomized trials with follow-up extending into and beyond the period of SVG failure. Therefore, at present, the concept of all arterial CAB surgery appears promising but awaits more reliable evidence [183, 184].
Ease of accessibility to the IMA and the LAD encouraged attempts at CAB surgery with nontraditional incisions and robotic assistance, and foregoing cardiopulmonary bypass [172]. Increasing experience led to the proposal for bypassing any or all territories without the assistance of cardiopulmonary bypass. Performing a distal anastomosis on a beating heart, whether by hand or with robotic assistance, without the traditional exposure gained through median sternotomy, is associated with a “learning curve” for the surgeon and some concern about the reliability of anastomoses to the distal circumflex and right coronary territories. Additionally, the use of special devices to assist in the construction of the anastomoses clearly reduces graft patency [185, 186]. Off-pump CAB surgery with traditional exposure and anastomosis techniques is generally equivalent in terms of outcome to the standard procedure, although, perhaps, a learning curve has influenced prior comparisons [187–192]. Avoiding cardiopulmonary bypass may reduce the postoperative inflammatory response, decrease perioperative morbidity, and improve outcomes for specific patient subsets, but in the low- to intermediate-risk patient, no benefit is apparent [192–195].
Patient Management
After a patient’s need for CAB surgery is confirmed, the target vessels for bypass and the urgency of the procedure must be decided and appropriate patient preparation begun. Complete revascularization is the goal. Every reasonably sized coronary artery (>1.5 mm) with a lesion causing ≥70 % diameter stenosis should receive a bypass. A caveat to this rule is that coronary artery size may be underestimated if there is complete vessel occlusion and poor collateral filling. Not infrequently, such a vessel that is initially judged to be too small or otherwise an inadequate target for bypass anastomosis is found to be a good target on visual inspection at the time of surgery. In addition, the accuracy of estimating anatomic stenosis severity solely from angiographic assessment has been called into question for both surgical and percutaneous revascularization. A visual assessment may not correlate with physiological assessment [196]. An abundance of accumulating evidence supports the concept that no anatomic diagnosis should be considered final without physiologic evidence to support the contention of severity. In fact, many patients may have their disease reclassified if physiologic testing is performed in connection with angiography. Such testing, such as nuclear scintigraphy, exercise echocardiography, and fractional flow reserve testing of individual arteries, is a necessary component of evaluating the necessity for revascularization and selecting the most appropriate procedure.
The disease presentation is the primary determinant of procedural urgency. A patient with evolving acute MI and symptom onset within the past 6 h may be referred emergently in order to abort the infarction, but even in these rare, optimal circumstances, the risk of perioperative complications is increased and alternative reperfusion methods may be more prudent. Realization of the need for surgery usually occurs after this time window, and the increased risk of perioperative complications posed by performing surgery within 1 week of the event mandates elective surgery after 1–3 weeks of medical therapy. Most often, urgent or emergent surgical referral is the result of procedural complications during percutaneous revascularization or mechanical complications of MI. Patients presenting with acute coronary syndromes and severe LMCA stenosis are at increased risk for progression to infarction in the 24 h after angiography and may warrant referral for urgent surgery [197]. In the majority of patients who are stable at the time of diagnosis, there is no indication for urgent or emergent surgery, although the reasonable upper limit of the waiting period has not been established.
Common complications of CAB surgery—including provoking a nonspecific inflammatory response, ventilatory failure, postoperative pump failure, atrial fibrillation, and graft thrombosis—may be avoided or ameliorated by proper patient preparation and adjunctive medical therapy. Pulmonary edema should be treated before the surgical procedure to ensure optimal volume state. During the procedure, pump-related hemodilution and increased capillary permeability will necessitate the administration of a large volume of crystalloid. The patient already in pulmonary edema when proceeding to surgery will require prolonged ventilator assistance and have an increased risk of pulmonary complications. Similarly, an exacerbation of chronic obstructive pulmonary disease should, in the best of circumstances, be treated to its resolution before surgery. Optimally, steroid therapy, if necessary, should be completed in order to avoid the increased risk of infectious complications.
The most important targets of preoperative medical therapy are perioperative myocardial ischemia and the nonspecific inflammatory response engendered by the surgical procedure and by cardiopulmonary bypass [198]. Cytokine generation from exposure to foreign membranes of the pump-oxygenator and perhaps bacterial translocation due to intestinal ischemia or edema may initiate an inflammatory response that, although generally short-lived, in some instances may be prolonged and severe [199–201]. Manifestations in the early postoperative period include fever, increased capillary permeability, non-cardiogenic pulmonary edema, coagulopathy, and low cardiac output due to myocardial stunning. Later manifestations include serositis, atrial fibrillation, renal failure, and encephalopathy. Although the incidence of most complications is most strongly correlated with preoperative risk variables such as age and comorbidity and with the duration of the surgical procedure, preoperative medical preparation can be beneficial. Beta-adrenergic antagonists, statin cholesterol-lowering drugs, the antiarrhythmic drugs sotalol and amiodarone, and perhaps angiotensin converting enzyme-inhibitors can reduce the risk of postoperative complications.
Atrial fibrillation is the most common complication of CAB surgery; its incidence is 25–30 %. It is seen with greater frequency in the elderly and patients with concomitant valvular heart disease or a history of the arrhythmia. Its occurrence is associated with an increased length of stay, risk of stroke, and long-term risk of death [202, 203]. Preoperative administration of beta-adrenergic antagonists, angiotensin converting enzyme-inhibitors, anti-arrhythmic drugs such as sotalol and amiodarone, and perhaps statins reduces the incidence of atrial fibrillation [204–207]. Amiodarone may be used with a brief period of oral loading preoperatively or IV loading at the time of surgery; maintenance therapy is continued only during hospitalization [206, 208]. Many physicians are reticient to use a drug with far-reaching effects, such as amiodarone. A less caustic and more anti-inflammatory option has recently been reported. Colchicine, started on day 3 after surgery and continued for 1 month, effectively halves the risk of postoperative atrial fibrillation when used in addition to other standard therapies [209].
Operative interventions for myocardial protection and limiting the inflammatory response include the use of blood cardioplegia, leukocyte depletion, and aprotinin [210]. Preoperative medical therapies with proven benefit include the beta-adrenergic antagonists and statin cholesterol-lowering drugs. Beta-adrenergic antagonists blunt the impact of perioperative ischemia and catecholamine release. In addition to a reduction in the incidence of postoperative atrial fibrillation, beta-blocker use is associated with a more rapid recovery of LV systolic function and improved operative survival [211–214]. Through uncertain mechanisms, beta-blockers may also reduce the risk of postoperative neurologic complications [215]. Similarly, angiotensin converting enzyme-inhibition may blunt the inflammatory response and reduce stunning/injury and the incidence of postoperative atrial fibrillation, although this is not firmly established [204, 216–218]. Statin cholesterol-lowering drugs, in addition to their benefit in lowering low-density lipoprotein concentrations, alter some components of the inflammatory response [219, 220]. Through this mechanism and perhaps others, statin drugs given preoperatively reduce the incidence of postoperative complications, including atrial fibrillation, sternal wound infection, and death [221–226].
Perioperative graft thrombosis is associated with MI and an increased risk of postoperative angina recurrence. Aspirin administration is associated with a reduced risk of SVG thrombosis and death [227]. In many patients with acute coronary syndromes, aspirin is administered preoperatively. In those patients with clinically stable disease not receiving aspirin before surgery, its use should begin within 6 h of the procedure. The use of thienopyridines in conjunction with aspirin is associated with an increased risk for bleeding complications and transfusion requirements (red blood cells and platelets) [228, 229]. Therefore, thienopyridines should be discontinued at least 5 days before planned surgery. Their value in conjunction with aspirin in the postoperative period has not been clarified.
Lastly, in the diabetic or glucose intolerant patient referred for surgery, perioperative glucose control is an important determinant of procedure related complications and survival [230]. Rigorously maintaining glucose concentrations <200 mg/dl, preferably by using continuous insulin infusions, is associated with >50 % reduction in the risk of death or deep sternal wound infection [231–233].
In the patient destined to undergo CAB surgery, it is our practice to ensure preoperative administration of a beta-adrenergic antagonist, an angiotensin converting enzyme inhibitor, and a statin in the absence of contraindications. In populations with an increased risk of postoperative atrial fibrillation, anti-arrhythmic drug therapy is considered preoperatively and, if used, is continued during hospitalization and for 1 month postoperatively in patients who develop the rhythm despite prophylaxis. The necessity of warfarin anticoagulation for patients who develop postoperative atrial fibrillation is not certain. It is our practice to provide warfarin anticoagulation for 3 months after surgery in patients with characteristics suggesting an increased risk of stroke, including a CHADS2Vasc score >2 and coexistent valvular heart disease.
Summary of Coronary Bypass Surgery
Patients with stable coronary disease, physiologic evidence confirming the significance of coronary lesions, and large territories of myocardium dependent on severely diseased coronary arteries benefit from revascularization regardless of symptom severity. Providing alternate routes for perfusing the myocardium at risk diminishes the symptoms of myocardial ischemia, limits the extent of muscle injury at the time of infarction, and provides, albeit temporarily, a survival benefit to patients with the most severe disease [80, 84, 85, 100, 104, 130, 131]. Patients who have undergone CAB surgery suffer less extensive MI at the time of subsequent disease progression. This seems to be the primary mechanism by which revascularization, applied in patients with stable CAD, may afford a survival benefit [130, 131].
Since the time of the randomized trials, improvements in perioperative care and the routine use of the IMA as a bypass conduit to the LAD have almost certainly expanded the population of patients for whom CAB is likely to improve survival. Even patients with the most severe ventricular dysfunction, who would not have been allowed entry into the randomized trials, have since been shown to benefit from surgical revascularization [234, 235]. Further improvements can be expected from modifications in surgical technique and medical therapy intended to reduce morbidity and improve long-term graft performance. However, in patients without proximal, major-vessel stenosis or in those whose anatomy does not allow use of the IMA, early surgical referral carries a stiff penalty when native or graft disease later mandates additional revascularization efforts. Therefore, although surgery is clearly beneficial in patients with severe coronary obstruction, those with less severe disease and other treatment alternatives should delay CAB as long as possible.
Percutaneous Revascularization
History
The successes of surgical revascularization, the limits on its application imposed by procedural risk, and engineering advances in catheter technology inevitably stimulated interest in nonsurgical revascularization methods (Table 26.6) [8–10, 236–245]. Initial attempts applied to peripheral vessels were quite crude: coaxial dilators were forced through a region of vascular stenosis. In 1974, Andreas Gruentzig developed the double-lumen balloon catheter [246, 247] that was used successfully in large, peripheral arteries and later miniaturized for use in coronary arteries [241, 242, 246]. After the first successful percutaneous dilation of a human coronary artery in 1977, techniques evolved rapidly as new technology was applied to subselective catheters, guidewires, and balloon materials. In addition, miniaturized devices for directional atherectomy, rotational atherectomy, extraction atherectomy, and laser ablation have been developed, each finding a clinical niche [248–263].
Table 26.6
Development of percutaneous coronary revascularization
Physician | Year | Technique | Reference |
|---|---|---|---|
Forssman | 1929 | Cardiac catheterization | [236] |
Cournand, Richards, Sones, Abrams, and Judkins | 1945–1967 | Diagnostic catheterization technique and angiography | |
Dotter and Judkins | 1964 | Transluminal angioplasty | [240] |
1974 | Balloon catheter | [241] | |
Gruentzig | 1977 | Coronary angioplasty | [242] |
Sigwart, Gianturco-Roubin, Palmaz-Schatz | 1986–1987 | Coronary stent | |
Multiple | 2000 | Drug-eluting stent |
The most significant advance in percutaneous revascularization has been the coronary stent, an expandable metal buttress for the balloon-dilated vessel. The stent provides not only mechanical support, preventing early vascular recoil and closure, but also a platform for local drug delivery. The proliferation of techniques for expanding a coronary lumen has resulted in a change in nomenclature from the original percutaneous transluminal coronary angioplasty (PTCA) to percutaneous coronary intervention or revascularization (PCI or PCR).
Natural History of the Treated Vessel
Balloon angioplasty reliably increases vessel-lumen diameter. The chance of success depends on the clinical situation and the lesion approached (Table 26.7) [264–266]. In patients with stable angina, the mortality at 1 month from the procedure is only 1 % [267]. Patients with limited disease and successful angioplasty enjoy a good prognosis, including 5-year survival exceeding 95 % [267, 268].
Lesion characteristic | Odds ratio |
|---|---|
Nonchronic total occlusion | 4.74 |
Bifurcation/inability to protect side branch | 4.25 |
Degenerated saphenous vein graft | 4.18 |
Thrombus | 4.09 |
Angulation + moderate calcification | 4.44 |
Length >20 mm/diffuse disease | 2.77 |
Severe calcification | 2.19 |
Eccentricity | 2.12 |
In the best of circumstances, percutaneous angioplasty results in near-total resolution of coronary artery stenosis; appearing to compress a malleable atheroma, angioplasty leaves a vessel whose angiographic appearance is smooth and regular. This appearance is quite deceptive [269–278]. Balloon angioplasty transmits increased intraluminal pressures circumferentially to the brittle, atherosclerotic vessel. Intimal fracture allows further balloon expansion and an increase in luminal diameter [269–277]. This fracture may extend, forming a plane of dissection whose size is determined by the mechanical characteristics of the lesion. A dissection plane involving the intima-media border may displace diseased intima and occlude the vessel. In approximately 2–10 % of balloon angioplasty procedures, intimal dissection, thrombosis, and perhaps medial smooth muscle spasm combine to reocclude the treated vessel soon after balloon deflation [268, 279, 280]. This event, termed abrupt closure, may be successfully treated with repeat balloon inflation in about one half of such cases [280, 281]. Intractable abrupt closure may be treated by stent placement or emergency CAB surgery [279–287]. Even when percutaneous methods of managing abrupt closure are successful, patients are at increased risk of MI [287]. It is the specter of abrupt closure and MI or emergency bypass surgery and its attendant complications that historically limited the application of balloon angioplasty.
The treated lesion, devoid of endothelial protection and rich in thrombogenic material, attracts platelets and initiates the formation of thrombus. After a successful procedure, platelet accumulation and thrombus formation is limited and is believed to provide both the stimulus and the framework for colonization by specialized vascular smooth muscle cells [288, 289]. These cells synthesize connective tissue and reinforce the injured intima. The appearance of an abundance of vascular smooth muscle cells and newly formed connective tissue has been termed intimal hyperplasia. Eventually, the angioplasty site is recovered by endothelium, and intimal hyperplasia gives way to collagen-rich connective tissue. Surrounding media and adventitia that is also injured by the mechanical effects of angioplasty becomes fibrotic and may alter its conformation, or remodel, as the vessel heals.
Intimal hyperplasia may become bulky and extend well into the vessel lumen. The result is no different from the original obstructing atheroma. Additionally, the media and adventitia may contract with healing and reduce the absolute cross-sectional area of the vessel. These two processes, intimal hyperplasia and vessel contracture, are the primary processes that produce restenosis after angioplasty [289–291]. Restenosis (>50 % diameter stenosis during follow-up) occurs after 40–50 % of PTCA procedures, almost always within 6 months of the procedure [266, 292–297]. However, only one-fourth of patients report recurrent angina warranting investigation [268]. Patients with restenosis are at increased risk of MI and are more likely to require CAB surgery [298]. Restenosis almost certainly has an adverse effect on survival [267].
Attempts to avoid restenosis initially centered on patient and lesion selection. With the introduction of coronary stents, the risk of abrupt closure and one of the mechanisms of restenosis, remodeling, was addressed effectively. However, the placement of a stent paradoxically exacerbates intimal hyperplasia such that the long-term impact of stent placement on restenosis is relatively minor [299–301]. The use of a stent as a drug delivery platform has met with great success, substantially reducing intimal hyperplasia, the recurrence of ischemia, and the need for repeat revascularization [302–305].
One caveat to the use of drug-eluting stents (DESs) is that antiproliferative drugs and the polymers that they are released from may delay re-endothelialization, thus prolonging the period of periprocedural risk of stent-related thrombosis [306]. First-generation DESs, coated with sirolimus and paclitaxel, prolonged this risk, leading to a recommendation for prolonged (1-year) dual antiplatelet therapy (DAPT). Many physicians elected to continue DAPT indefinitely, particularly in patients with multivessel or complex disease. Newer stent platforms and antiproliferative drugs allow for earlier endothelialization and a somewhat shorter duration (6 months) of DAPT [307]. An even newer generation of absorbable stent platforms may change that calculus even further [308].
Randomized trials of early surgical revascularization are consistent in reporting that early surgical referral does not improve the survival of patients with 1- or 2-vessel disease and good ventricular function (noting the high rate of eventual surgical bypass in the “medically treated” patients). In these populations, the risk of adverse events associated with surgical bypass and the subsequent need for repeat procedures balances or may outweigh the benefit obtained from early referral for revascularization. Percutaneous procedures are associated with a lower risk of morbidity and are not associated with an increased risk should CAB surgery or a repeat percutaneous procedure become necessary. Therefore, PCR seems to be an ideal treatment for those patients with unacceptable symptoms for whom surgery will provide no clear survival benefit.
Impact of PCR on Survival
In part because most trials have not had enough patients or events to achieve substantial statistical power, no trial has established a survival benefit for PCR compared to medical therapy in patients with stable angina. Without the aggressive medical regimen that is used today, a CAD patient could expect a risk of fatal coronary events of about 3 %/vessel/year [27]. If a successful balloon angioplasty procedure were to reduce the likelihood of death with the next event to that of patients with no occlusive disease, or by about 30–40 %, and if slightly more than one-half of treated patients enjoy a durable success, the expected reduction in mortality is <20 %. In order to have an 80 % chance of detecting such a small effect in a group of patients with mortality as high as 3 %/year, a study of more than 10,000 patients with follow-up for 5 years would be necessary. Much smaller studies are often used to address the question of the impact of angioplasty on survival, an inappropriate use of their data [309–311]. Retrospective database studies have the benefit of not having to specifically identify patients for enrollment and, although subject to bias, may have greater statistical power. Findings from the Duke University database (N = 9263) suggest that revascularization therapy benefits all severities of coronary disease with PTCA, especially in cases of 1-vessel-region disease [312].
With the thought that improving treatment durability with modern stents might alter the relationship between PCI and medical therapy, the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation trial was performed. Enrolling 2,287 patients with a median of 4.6 years of follow-up, better control of angina was observed without benefit in terms of ischemic events [313]. Although no study has yet been adequately powered to address the question, the available evidence seems to support the principle uncovered in the surgical randomized trials that despite the availability of less morbid revascularization options, patients without high-risk anatomic and physiologic criteria can safely be treated medically unless symptom control is unacceptable.
Trials of PTCA Versus Medical Therapy
The Angioplasty Compared to Medicine trial evaluated the efficacy of PTCA as compared to medicine in the treatment of symptomatic 1-vessel CAD [309]. Entry criteria included a 70–90 % narrowing of a single coronary artery plus stable angina, a strikingly positive exercise test (≥3 mm ST segment depression or reversible thallium defect), or a history of MI within 3 months of enrollment. Two hundred and twelve patients were ultimately randomly assigned to either PTCA (n = 105) or medical therapy (n = 107). Follow-up continued through 6 months, when a scheduled exercise test was performed. The primary end points of the study were change in exercise tolerance from baseline, frequency of angina, and nitroglycerin usage.
Only 100 of the 105 patients randomly assigned to PTCA actually underwent the procedure, with a success rate of 80 %. Four PTCA patients experienced perioperative MI. There were no deaths. At 6 months, 1 patient who had been randomly assigned to medical therapy had died. Eight patients suffered an MI: 1 further patient in the PTCA group, to total 5, and 3 in the medicine group. Twenty-seven patients underwent PTCA: 16 repeat procedures in the PTCA group and 11 first procedures in the medicine group. Bypass surgery was required more often in patients randomly assigned to PTCA; 7 operations were performed (2 emergent), compared to none in the medical group. By 3 years of follow-up, 6.1 % of patients randomly assigned to medical therapy had died, compared to 4.8 % of patients who underwent PTCA (p = NS). The incidence of MI and CAB surgery was the same between groups [310].
Percutaneous transluminal coronary angioplasty was superior to medicine in relieving the symptoms of myocardial ischemia; 64 % of the PTCA patients were free of angina at 6 months, compared to 46 % of the medical patients. In addition, PTCA increased exercise duration by 1.6 min over medical therapy. Revascularized patients also enjoyed a substantial improvement in their overall sense of well-being and quality of life, and these self-reported data correlated with objective measures of ischemia [314]. The Angioplasty Compared to Medicine investigators added to these observations by studying a small group of patients with 2-vessel disease, finding no significant difference in follow-up events and a trend toward better control of angina with revascularization [315].
A second small study of 1-vessel revascularization was performed in patients who were asymptomatic or whose symptoms were easily controlled with medication [316]. Eighty-eight patients with a mean single coronary artery stenosis of 86 % were randomly assigned to PTCA (n = 44) or continued medical therapy (n = 44). Patients with a prior Q wave MI, a treadmill test positive at 50 W, or diabetes mellitus were excluded. Two-year follow-up data revealed no difference in survival.
The Randomized Intervention Treatment of Angina trial compared PTCA to medical therapy in 1018 patients with stable or unstable angina pectoris and no definite indication for CAB surgery or urgent revascularization [311]. Medical therapy included a beta-blocker and a calcium channel blocker or long-acting nitrate preparation. The use of lipid-lowering agents varied throughout the course of the study. At randomization, 20 % of patients had functional class 3 or 4 angina, 40 % had multivessel disease, and 45 % had moderate to severe LV wall-motion abnormalities. At 2.7 years’ follow-up, there was no difference in mortality between the groups. Myocardial infarction was seen twice as frequently in the PTCA group, so that at completion of follow-up, the incidence of death or MI was 6.3 % in the PTCA group, as compared to 3.3 % in the medically treated patients (p = 0.02). In contrast, revascularization was associated with better control of angina and a trend toward a decreased frequency of congestive heart failure.
Four trials have compared any form of revascularization to standard medical therapy: the Medicine Angioplasty or Surgery Study I and II trials, the ACIP (Asymptomatic Coronary Ischemia Pilot) study, and Trial of Invasive versus Medical Therapy in Elderly Patients [317–321]. Their results are consistent with the prevailing theme that revascularization improves symptoms of angina. Any hint of survival benefit is limited to patients with severe physiological limitation or angiographic disease extent, and the incidence of MI is virtually unaffected by revascularization [318–324].
The ACIP study was designed as a preliminary study seeking the most appropriate form of therapy for patients without definite indications for revascularization whose symptoms might be unreliable as a guide to ischemia severity. Patients with objective ischemia on physiologic study and at least 1 episode of asymptomatic ST-segment depression during 24-h ambulatory ECG recording were randomly assigned to medical therapy guided by symptoms, medical therapy guided by objective ischemia, or revascularization. The attending physician chose the mode of revascularization [319]. Sixteen percent of patients had diabetes mellitus, 40 % had a history of MI, and 76 % had multivessel disease. Of particular note, 35 % of patients had stenosis of the proximal LAD. At 2 years, mortality in patients receiving medical therapy guided by symptoms was 6.6 %. Ischemia-guided medical therapy and revascularization resulted in a mortality of 4.4 and 1.1 %, respectively [323].
The observed differences in survival in ACIP have several possible explanations, but the most interesting arises from an analysis of diagnostic angiograms used to screen patients. Patients who had silent ischemia meeting the criteria for study entry were more likely than other patients to have proximal, discrete, complex coronary lesions [324]. An in-depth angiographic analysis from the CASS registry had previously found the position of lesions (i.e., proximal or nonproximal) to be an important predictor of outcome (Fig. 26.9) [35]. For example, patients with proximal 2-vessel disease had survival that was virtually identical to that of patients with 3-vessel disease and no or 1 proximal stenosis. The observed survival of patients with nonproximal 1-vessel disease was virtually identical to that of patients with no disease. Therefore, the ACIP study population largely consisted of patients with multivessel coronary disease, many of whom had proximal stenoses that are associated with decreased survival expectancy and probable benefit from revascularization. Regardless of how the population may have been skewed, ACIP provides the first evidence that revascularization (not just CAB surgery) can improve the survival of patients with CAD who do not fit into the categories defined by the randomized surgical trials. The angiographic substudy adds to the findings of Ringqvist and coworkers [35], casting a shadow on the simple classification of number of vessels diseased.
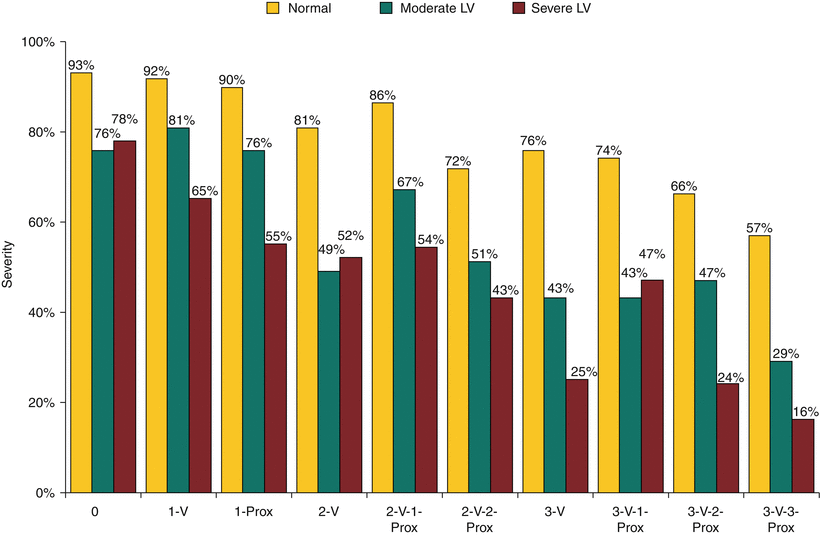
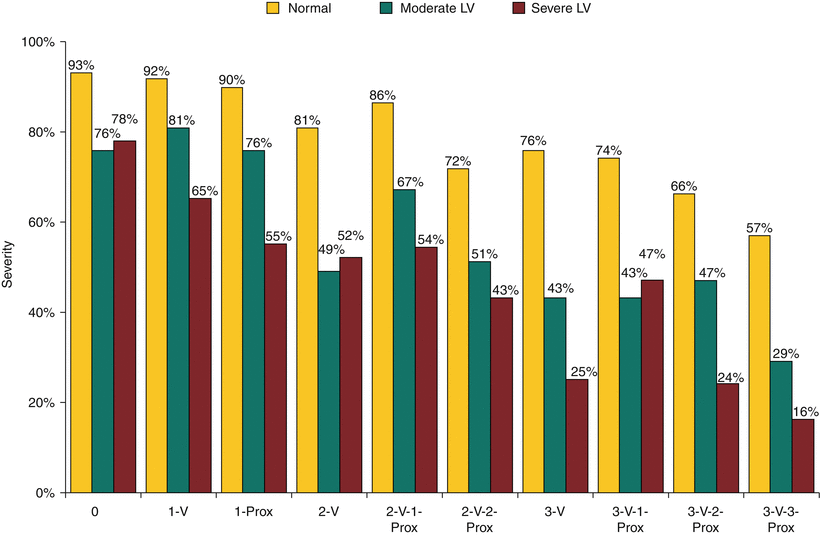
Fig. 26.9
Modified anatomic description of disease severity. The anatomic description of coronary disease severity should express the quantity of myocardium that is or may become ischemic under hemodynamic stress. An improvement on the first practical and successful scheme uses the number of affected major vessel regions with the position (proximal or nonproximal) of stenosis, as well as ventricular function (Source: Data from Ringqvist et al. [35])
Comparing PCR to Coronary Bypass Surgery
Many patients with symptomatic atherosclerosis have multivessel coronary disease and require revascularization for symptom control. The surgical trials defined select populations that are provided a survival advantage with surgical bypass. The magnitude of survival advantage declines with time because of the inherent limitations of non-IMA bypass conduits. Percutaneous revascularization is successful in reducing angina and may pose a lower risk of procedural morbidity and allow an earlier return to normal activity. The profound psychological and physical effects of bypass surgery and the finite life span of most bypass grafts favor percutaneous interventions, when feasible. However, patients with multivessel coronary disease in whom complete revascularization is not achieved may pay a price in terms of survival expectation, particularly when LV function is compromised, and percutaneous revascularization methods are often incapable of complete revascularization [325–327]. Several randomized trials have been performed to determine what impact, if any, the type of revascularization method has on survival outcome. Using a similar philosophy to that of the surgery versus medicine trials, in the event of clinical deterioration or the opinion of the attending cardiologist, patients randomly assigned to PCR could cross over to surgery, and many patients did (Table 26.8). The randomized trials of PTCA and surgery offered evidence that, in carefully selected patients with multivessel CAD who require revascularization predominantly for the control of angina, initial referral for PTCA can safely delay or prevent CAB with the caveat that PTCA-treated patients enjoy less complete revascularization and require more additional procedures [317, 318, 320, 328–334, 336–339, 341, 343–345, 348, 349]. The slight differences in completeness of revascularization and marked differences in the need for repeat procedures had no real impact on survival comparisons except in treated diabetic patients. In the largest of the trials, Bypass Angioplasty Revascularization Investigation, a slight mortality difference favoring surgery in the total population (84.4 % vs. 80.9 %, p = 0.043) was almost completely accounted for by the effect of LIMA-LAD bypass in treated diabetic patients. The 7-year survival of patients receiving an IMA graft was much better than patients receiving only SVGs, whose observed survival was no different from that of patients undergoing PTCA (LIMA 83.2 %, CAB-SVG ONLY 54.5 %, PTCA 55.5 %) [335].
Table 26.8
Randomized comparisons of percutaneous coronary revascularization (PCR) to coronary artery bypass (CAB) surgery
Trial | Disease extent | Follow-up period (year) | Tx | n | Procedural risk | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Death | MI | ER | Death | MI | CABG | PTCA | ||||||
RITA-1 | >50 % stenosis of ≥1 coronary artery with an equal chance of successful revascularization | 6.5 | PTCA | 510 | 1.8 % | 4.3 % | 7.6 % | 10.5 % | 26.3 % | 27.1 % | ||
CABG | 501 | 1.2 % | 9 % | 7.4 % | 2.8 % | 9.4 % | ||||||
GABI [328] | >70 % stenosis of >1 coronary artery; severe angina | 1 | PTCA | 176 | 1.1 % | 2.3 % | 11.4 % | 0.6 % | 6 %a | 18 % | 23 % | |
CABG | 161 | 2.5 % | 8.1 % | 1.9 % | 0.7 % | 13.6 %a | 1.0 % | 5.0 % | ||||
>70 % stenosis of ≥1 coronary artery; revascularization judged necessary | 3 | PTCA | 63 | 1.5 % | 6.3 % | 1.5 % | 9.5 % | 7.8 % | 22.2 % | 14.5 % | ||
CABG | 64 | 4.6 % | 6.2 % | 1.5 % | 4.7 % | 7.8 % | 0 % | 4.9 % | ||||
MASS [317] | >80 % proximal LAD stenosis, low-risk lesion; normal LVEF | 5 | PTCA | 72 | 0 % | 1.3 % | 1.3 % | 5.7 % | 11.1 % | 30.3 % | ||
CABG | 70 | 0 % | 1.3 % | 0 % | 2.9 % | 5.7 % | 0 % | 0 % | ||||
>50 % stenosis in ≥2 vessels with equal chance of successful revascularization | 8 | PTCA | 198 | 1 % | 3.0 % | 10 % | 20.7 % | 14.6 % | 29.3 % | 51 % | ||
CABG | 194 | 1 % | 10.3 % | 0 % | 17.3 % | 19.6 % | 2.4 % | 24.6 % | ||||
CABRI [333] | 2- or 3-vessel disease, with ≥1 vessel suitable for angioplasty | 1 | PTCA | 541 | 1.3 % | NR | NR | 3.9 % | 4.9 % | 15.7 % | 20.8 % | |
CABG | 513 | 1.3 % | NR | NR | 2.7 % | 3.5 % | 0.8 % | 2.7 % | ||||
>50 % stenosis of >1 coronary artery + angina/MI | 7 | PTCA | 904 | 1.1 % | 2.1 % | 8.4 % | 19.1 % | 26.5 %a | 35.5 % | 37.2 % | ||
CABG | 892 | 1.3 % | 4.6 % | 0.1 % | 15.6 % | 24.7 %a | 1.4 % | 12.1 % | ||||
French Monocentric [336] | ≥70 % stenosis of >1 coronary artery with + TMT/MI | 5 | PTCA | 76 | 1.3 % | 3.9 % | 3.9 % | 13.2 % | 6 %b | 13.6 % | 15.2 % | |
CABG | 76 | 1.3 % | 6.6 % | 1.3 % | 10.5 % | 1.5 %b | 0 % | 4.4 % | ||||
MASS-II [320] | ≥70 % of ≥2 coronary arteries with preserved LV function and suitability for PTCA | 1 | PTCA | 205 | 2.4 % | 1.5 % | 4.4 % | 8.3 % | 3.9 % | 8.3 % | ||
CABG | 203 | 3 % | 2 % | 3.9 % | 3.0 % | 0 % | 0.5 % | |||||
AWESOME [337] | Refractory ischemia + high risk for surgical complication; angioplasty or stenting | 3 | PCR | 222 | 3 % | 2 % | 20 % | 11 % | ||||
CABG | 232 | 5 % | 0 % | 21 % | 4 % | |||||||
Equal suitability for stent or surgical revascularization; multivessel disease; LVEF >30 % | 5 | Stent | 600 | 6.2 % | 2.3 % | 8 % | 6.7 % | 10.5 % | 23.2 % | |||
CABG | 605 | 12.6 % | 0.3 % | 7.6 % | 5.6 % | 1.2 % | 8.3 % | |||||
SoS [341] | Multivessel CAD equally suitable for stenting or surgical revascularization; normal LVEF | 2 | Stent | 488 | 1 % | 5 % | 5 % | 9 % | 13 % | |||
CABG | 500 | 4.8 % | 2 % | 8 % | 1 % | 5 % | ||||||
ERACI-II [342] | Multivessel CAD equally suitable for stenting or surgical revascularization. | 1 | Stent | 225 | 0.9 % | 0.9 % | 0.4 % | 3.1 % | 2.3 % | 16.8 % | ||
CABG | 225 | 5.7 % | 5.7 % | 0 % | 7.5 % | 6.3 % | 4.8 % | |||||
Utrecht [343] | ≥1-Vessel CAD referred for PCI | 1 | Stent | 138 | 0 % | 2.2 % | 3.6 % | 0 % | 4.4 % | 4.4 % | 10.9 % | |
OPCAB | 142 | 0.7 % | 4.9 % | 2.1 % | 2.8 % | 4.9 % | 0.7 % | 3.5 % | ||||
Groningen [343] | “High Grade” PLAD | 2.9 | Stent | 51 | 0 % | 0 % | 9.8 % | 15.7 % | ||||
Type B2 or C lesion | OPCAB | 51 | 4 % | 5 % | 4 % | 2 % | 4 % | |||||
SIMA [344] | PLAD stenosis | 2.4 | Stent | 62 | 2 % | 4 % | 1.6 % | 2 % | 5 % | 6 % | 13 % | |
LVEF >45 % | LIMA | 54 | 0 % | 1.9 % | 0 % | 2 % | 4 % | 0 % | 0 % | |||
Leipzig [345] | >75 % focal stenosis of PLAD | 0.5 | Stent | 110 | 0 % | 1.8 % | 1.8 % | 1.8 % | 2.7 % | 3.6 % | 23 % | |
OPCAB | 110 | 1.8 % | 3.6 % | 3.6 % | 1.8 % | 4.5 % | 0 % | 4.5 % | ||||
Freedom [346] | 70 % stenosis in >2 vessels | 3.8 | PCI | 953 | 0.8 % | 1.8 % | 3.3 % | 16.3 % | 13.9 % | NR | ||
CABG | 947 | 1.7 % | 1.7 % | 1.1 % | 10.9 % | 6.0 % | NR | |||||
>50 % stenosis in 3 vessels or LMCA | 5 | PCI | 903 | 0.1 % | 0.2 % | 13.9 % | 9.7 % | 25.9 % | ||||
CABG | 897 | 0.2 % | 0.4 % | 11.4 % | 3.8 % | 13.7 % | ||||||
PRECOMBAT [347] | >50 % stenosis of LMCA | 2 | PCI | 300 | 1.3 %c | |||||||
CABG | 300 | 3.0 %c | ||||||||||
Boudriot [348] | >50 % stenosis of LMCA | 1 | PCI | 100 | 3.0 % | 1 % | ||||||
CABG | 101 | 3.0 % | 2 % | |||||||||
Stents
By reducing the likelihood of abrupt closure that necessitates emergency CAB surgery and the occurrence of restenosis, stent-assisted angioplasty is more effective than routine balloon angioplasty for treating virtually any type of coronary artery lesion. However, because of the option for “bail-out” stent implantation in randomized comparisons to PTCA, a procedure using coronary stents has not been proven to be safer than balloon angioplasty. Registry data are more favorable, describing a risk for emergency surgical referral of only 0.3–1.1 % and a procedural mortality of <1 %, even in patients whose disease severity suggests high procedural risk [350, 351]. The use of stents improves the durability of the revascularization procedure, reducing the risk of short-term failure by 30 % or more, but no difference in survival has been observed [299, 300, 352–360]. Long-term follow-up studies of stent-PCR suggest that, unlike SVGs, sites within a native artery treated by stent revascularization are not subject to late, rapid disease progression, and that their benefit is long lasting [361–365]. But, after 5 years, 10–15 % of stent-treated patients require another revascularization procedure for severe stenosis at a non-stented site [306, 362]. Diabetes remains an important risk factor for adverse outcome because it confers an increased risk of restenosis and of progression of disease at untreated sites [362, 364, 366–369]. With stent-PCR showing no effect on survival in comparison to PTCA [370], comparisons between stent-PCR and CAB have differed only slightly from the earlier comparisons between PTCA and CAB (Table 26.8) [337, 338, 340–342, 367, 371].
The risk of angiographic restenosis and need for repeat revascularization procedures is profoundly reduced after the implantation of stents eluting antiproliferative drugs in almost any clinical setting where stent implantation is considered (Table 26.9) [303, 372–387]. Studies examining the efficacy of DESs introduced a new nomenclature regarding the endpoints for follow-up. The most useful follow-up endpoint is termed target-vessel failure and consists of cardiac death, MI, or repeat revascularization of the treated vessel. At 1 year after the procedure, the rate of target-vessel failure is reduced from 19.4–21 % with a bare-metal stent to 8.8–10 % with a DES [303, 372]. Similar relative reductions in this endpoint are reported for every type of lesion studied, suggesting that DESs are the standard of care for a percutaneous revascularization procedure. However, the impact of DESs on the risk of long-term treatment failure and repeat procedures does not translate to a reduced risk of procedure-related complications [388]. Therefore, there is no anticipated change in the balance of risk and benefit that determines the threshold for a revascularization procedure.
Table 26.9
The clinical impact of drug-eluting stents (DESs)
Population | End point | Bare-metal stent (%) | DES (%) |
|---|---|---|---|
TVF | 20–24.1 | 9.9–10.8 | |
MACE 9 months | 27.2–36.3 | 11.3–15 | |
Insulin treated [373] | MACE 9 months | 31.5 | 19.6 |
Myocardial infarction [376] | TVR 8 months | 32 | 18 |
Complex lesions [377] | TLR 12 months | 29.8 | 2.4 |
MACE 9 months | 18.3–22.6 | 4–8 | |
Small vessel [381] | MACE 8 months | 31.3 | 9.3 |
TVR 6 months | 13.3–38 | 8.6–19 | |
Restenosis [386] | TVR 6 months | 33 | 8–19 |
Saphenous vein graft lesion [387] | MACE 6 months | 28.1 | 11.5 |
Available evidence concerning balloon angioplasty, stent revascularization, and CAB surgery has established that the potential benefit of treatment is proportional to the probability of death with the next MI or, in more measurable terms, the amount of ischemic myocardium revascularized (Fig. 26.10) [389]. Comparisons to ensure that a treatment strategy beginning with PCR-stent can afford a similar outcome to early referral for surgery have been favorable in selected patient populations. However, since the publication of the SYNTAX trial, clinically defined populations have waned in importance.
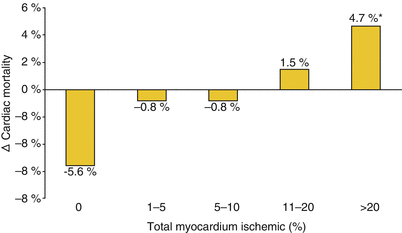

Fig. 26.10
The threshold for revascularization. Hachamovitch et al. [389] examined the short-term survival benefit of revascularization in comparison to medical therapy in a cohort of 10,627 patients without a history of myocardial infarction or revascularization who underwent exercise or adenosine perfusion scintigraphy. The amount of inducible ischemia was proportional to mortality in patients undergoing medical therapy but not in patients referred for revascularization. The difference between the two suggests that a survival benefit from revascularization appears at a threshold between 10 and 20 % of ischemic myocardium
Specific risk factors associated with the superiority of surgical revascularization over PCR-stent for a particular patient, such as diabetes mellitus and renal failure, are also associated with an increase in the number and complexity of coronary artery lesions likely to require treatment. With greater complexity comes greater risk of procedure-related complications and long-term treatment failure. Conversely, the absence of these risk factors cannot guarantee the absence of complexity. Therefore, Serruys and coworkers used a randomized trial of DES versus CAB surgery to examine the predictors of procedural failure, both short and long term, in reverse. Using an angiographic core lab, they examined the anatomic predictors of procedural failure only, later adding clinical data. Each anatomic predictor was give a statistical “weight”. This allowed derivation of a prediction score, the Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX score), that has proved extraordinarily useful. In fact, this score represents the most important advance in the classification of CAD since Proudfit’s original publication of Mason Sones’ data (for the latest version of the SYNTAX score calculator, see http://syntaxscore.com) (Table 26.2) [390, 391].
Many of the risk factors identifying surgery as the superior form of therapy are associated with an increased risk of recurrent ischemic events during follow-up. In fact, after the early recovery period, recognized risk factors for the development of CAD are the primary drivers of outcome [392]. Which of the important clinical risk factors will remain important after anatomic assessment remains a work in progress. For example, although diabetes is no longer factored into the clinical score, diabetes remains important in determining long-term outcome [393].
Diabetes Mellitus
The risk of MI, MI-related death, restenosis, or complete vessel occlusion after angioplasty or bypass graft failure is substantially increased in patients with diabetes [366, 394–400]. These risks are related to the duration of diabetes and the severity of diabetic end-organ damage [401]. The mortality in diabetic patients undergoing CAB surgery is greater than the non-diabetic population (4.2 % vs. 1.8 %). After successful revascularization, the 5-year survival adjusted for various risk factors is 75 %, with 2.2 % requiring reoperation [402]. Percutaneous treatment of multivessel disease in diabetics is associated with a similarly adjusted 5-year survival of 68 % [402]. After coronary stent placement, the mortality observed at 30 days is nearly doubled (2.7 % vs. 1.4 %) by the presence of diabetes mellitus [366]. In long-term follow-up after stent placement, mortality adjusted for risk factors remains elevated (6.6 % vs. 4 %). Stent restenosis occurs with a frequency of 37.5 % with 21.1 % requiring repeat revascularization [366].
Diabetic patients tend to have more diffuse atherosclerosis and a greater prevalence of additional lesions in the treated vessel than nondiabetic patients [403, 404]. Noncritical stenoses have prognostic significance, representing sites within the vessel that are severely diseased and have the potential for rapid evolution [26]. In a vessel with multiple noncritical lesions in addition to a severe stenosis, the ability of percutaneous therapy to provide reliable, complete revascularization may be impaired [405]. In fact, angiographic evidence of the extent of atherosclerosis may be more important than the diagnosis of diabetes mellitus in determining the long-term success of percutaneous revascularization.
Detre et al. [406] examined the effect of CAB surgery and PTCA on mortality after MI in both the randomized-trial and registry populations from Bypass Angioplasty Revascularization Investigation. Diabetic patients suffered an almost twofold increase in the risk of Q-wave MI [407], whose incidence was not related to the procedure chosen. In the event of such an MI, 5-year mortality was 17 % in patients who underwent CAB surgery and an amazing 80 % in those who did not. Any patient who underwent CAB surgery at any time after entry into the study was considered to be in the CAB group. After 5 years, 64 % of diabetic patients initially treated with PTCA underwent CAB surgery. Therefore, it is possible that bias, or “surgery for the fittest,” results in overestimation of the relative impact of CAB surgery. However, it is very unlikely that bias is responsible for all of the rather dramatic differences between the groups. More likely, it is the result of less effective revascularization with PTCA [408]. Unfortunately, DESs have not produced the reduction in restenosis rates that was hoped to change this calculus. The results of the Freedom trial of DES similarly favored surgical therapy. However, SYNTAX may trump these observations [346].
The Bypass Angioplasty Revascularization Investigation 2 Diabetes trial, which included 2,364 patients, examined the outcomes of an initial strategy of coronary revascularization and optimal medical treatment compared with optimal medical treatment with the option of subsequent revascularization. Early revascularization improved angina control but had no effect on important outcomes, thus returning us to our initial principle of using physiologic risk combined with lesion complexity as the central point of decision-making when treating patients with CAD.
Left Main Coronary Artery Disease
Treating left main CAD has historically been within the purview of CAB surgery. However, specific lesion subtypes offer the possibility of a reasonable procedural risk with reliable long-term outcome. There have been numerous studies conducted in southeast Asia [347], but the natural history of PCI-treated CAD there may differ from that of westernized countries [409]. However, trials in westernized nations have been equally promising.
In the SYNTAX trial, patients with a low score had limited disease, and PCI performance was reasonable in comparison to surgery. In patients with more complex disease, surgery appeared to maintain superiority [46, 410]. In short, there is a role for PCI in the treatment of LMCA stenosis, but that role is as yet not clearly defined and will be restricted to patients with non-complex disease.
Patient Management
As with surgery, the frequency of complications associated with percutaneous revascularization may be reduced by adjuvant medical therapy. Principal among these complications are periprocedural MI and radiocontrast nephropathy. Q-wave MI complicates <1 % of routine PCR procedures. However, a procedure-associated rise in CK-MB, suggesting myocardial necrosis, occurs with an incidence of almost 20 %. An asymptomatic rise in cardiac markers after a revascularization procedure alters both short- and long-term prognosis in proportion to the number and severity of the patient’s comorbidities [411, 412]. The risk is dependent on the clinical setting, disease severity, and lesion characteristics [287, 412]. Mechanisms of infarction include ischemia during abrupt closure, side-branch closure, and embolization of thrombus and perhaps cholesterol crystals. Glycoprotein IIb/IIIa antagonists are protective when used during and after the procedure. Pretreatment with the combination of aspirin and a thienopyridine antagonist may obviate the use of glycoprotein IIb/IIIa antagonists (Table 26.10) [414, 415]. In addition, statin cholesterol-lowering therapy is associated with a reduced risk of procedural MI even when instituted in close temporal proximity to the procedure [416, 417].
Table 26.10
Coronary artery bypass graft surgery mortality in patients with acute myocardial infarction (MI) [413]
Time from infarction | Mortality (%) | ||
|---|---|---|---|
Q-wave MI | Non-Q-wave MI | Total mortality | |
<48 h | 50 | 0 | 18 |
3–5 days | 0 | 16 | 3.3 |
6–42 days | 10 | 1.9 | 2.2 |
The mechanism of radiocontrast nephropathy (RCN) is not known. Its occurrence is a function of age, congestive heart failure, hemodynamic instability, diabetes mellitus, pre-existing renal insufficiency, anemia, and the amount of contrast administered [418–420]. When defined as a >25 % or >0.5-mg/dL rise in serum creatinine concentration, the incidence of RCN after coronary angiography is 5–6 %, with the nadir of renal function occurring 3–5 days after the procedure [421, 422]. In patients at high risk, the incidence may be more than 20 % [423]. Even a transient decline in renal function is associated with an increased risk of ischemic cardiovascular events during follow-up, and renal failure necessitating hemodialysis, which is seen in about 10 % of patients with RCN, substantially increases both short- and long-term mortality risk [418, 420, 424]. Attempts to limit the incidence of RCN have included using of calcium channel antagonists, aminophylline, acetylcysteine, fenoldopam, dopamine, atrial natriuretic peptide, sodium bicarbonate, hemofiltration, and iso-osmolar and non-ionic contrast agents, and maintaining brisk urine flow with crystalloid and diuretics [422, 423, 425–432]. With some exceptions, trials of these agents have been disappointing. Iodixanol, an iso-osmolar, non-ionic contrast agent, reduced the incidence of RCN after noncardiac angiography [432]. N-acetylcysteine given in 4 oral doses of 600 mg, 2 the day before and 2 the day of the procedure, has produced various outcomes in several studies [427, 433–435]. The most uniformly effective intervention is ensuring an adequate volume state by administering crystalloid in volumes sufficient to maintain brisk urine flow [422, 423]. Additionally, alkalinization of urine pH may increase the effectiveness of fluid administration. The administration of sodium bicarbonate solution at a dose of 3 mL/kg/h for 1 h before contrast and 1 mL/kg/h for 6 h after the procedure reduced the incidence of RCN from 13.6 to 1.7 % [426]. Forced diuresis with diuretics is of minimal or no additional value, and the use of dopamine to reduce the likelihood or duration of RCN may be harmful [423, 430].
Acute Coronary Syndromes
Coronary artery atherosclerotic lesions have the potential to undergo rapid, thrombotic evolution, abruptly altering the dynamics of coronary blood flow [436–448]. Complete vessel occlusion or severe narrowing may result in acute MI and, in some instances, sudden death [438–441, 449, 450]. Restoring flow rapidly resolves symptoms and improves short- and long-term complication-free survival [451–464].
Realization of the importance of atheroma evolution and thrombus formation in unstable coronary syndromes has led to the use of aggressive antiplatelet and anticoagulation strategies and, in specific circumstances, the administration of thrombolytic agents. All have been remarkably successful in treating unstable angina and MI. Although heparin and aspirin are effective therapies for unstable angina pectoris, they do not dissolve existing thrombus. In some patients, they are unable to prevent the progression to MI [465]. Thrombolytics are not uniformly effective. They confer a significant risk of hemorrhage, do not address the underlying coronary stenosis, and have no proven value in patients without ST-segment elevation or new left bundle branch block [459, 461, 466–468]. Therefore, a prominent role remains for mechanical revascularization in the therapy of unstable coronary syndromes [464].
Surgical bypass reduces mortality and the extent of myocardial damage when employed in evolving (<6 h) MI [451, 453]. Indeed, this was once not an uncommon reason for emergency bypass surgery, the inciting event being failed PTCA. Surgical mortality is increased, ranging from 2.9 to 38 % in patients with cardiogenic shock necessitating inotropic or intra-aortic balloon pump support [453, 462]. Furthermore, the benefit of surgery, like that of thrombolysis, may be realized only if revascularization is achieved early [451, 453]. Emergency surgery may be performed with relative safety despite prior administration of thrombolytic agents [463]. There is, of course, an increased risk of hemorrhage and transfusion requirement. However, limited experience suggests that only 8 % of patients so treated suffer severe effects (warranting re-exploration to search for a source of bleeding) [463].
When surgery is performed beyond the first 6 h in patients with acute MI, the risk of death or complications is substantially increased (Table 26.10) [413]. After 1–6 weeks, the risk of adverse events returns to normal. Therefore, in a patient who is stable after MI but whose anatomy warrants referral for CAB surgery, the procedure should be delayed for at least 1 week and perhaps longer if possible.
Surgical bypass is effective in relieving ischemic symptoms and improving the survival of patients with refractory unstable angina pectoris [462, 469–472]. Operative mortality is increased in patients with unstable angina or with LMCA or 3-vessel CAD [462, 469, 470]. Although the quoted perioperative mortality ranges from 1.7 to 5.7 %, improvements in supportive care have produced a decline in surgical deaths [453, 462, 469, 470, 473]. Similar to therapy for stable angina pectoris, surgical revascularization does not confer a survival benefit to patients with 1- and 2-vessel CAD and normal LVEF [462, 472]. In patients with impaired ventricular function, the benefits are profound. Medically treated patients with an LVEF <50 % have a mortality of 17.6 % at 3 years, compared to 6.1 % in patients who undergo bypass surgery.
Percutaneous transluminal coronary angioplasty is a successful mode of therapy for acute coronary syndromes [452, 454–456, 474–491]. Successful PTCA within the first 6 h of ST-elevation MI is as effective as thrombolytic agents in limiting myocardial damage and improving in-hospital survival [452, 454, 455, 474, 475, 492, 493]. Beyond treating the intracoronary thrombus that is so often present, PTCA addresses the underlying coronary artery stenosis. The incidence of recurrent ischemia is reduced, LV function may be better preserved, and survival is probably better than that of patients treated with thrombolytic agents [454, 455, 477, 492, 494, 495]. Furthermore, PTCA does not incur a significant risk of intracranial hemorrhage [455]. Although direct angioplasty is successful in most cases, failure due to inability to address the culprit vessel or due to impaired reperfusion at the level of microscopic vessels is associated with an increased risk of mortality [496–498]. Nonetheless, early angiography and revascularization substantially improve the likelihood of survival in patients with shock complicating MI [499].
Patients who undergo urgent PTCA have a 20–40 % risk of recurrent angina, restenosis, or the need for repeat revascularization. Stenting, which was initially avoided in arteries with a high probability of harboring thrombus, is more effective than PTCA alone, especially when used in combination with a potent antiplatelet regimen [358, 500–513]. Stenting has compared favorably with thrombolytic therapy in both early and late outcome [474, 495, 514, 515]. Drug-eluting stents perform even better because of the lower risk of late treatment failure [516]. In patients with acute MI, the superiority of stent-PCR to thrombolytic therapy is such that a delay of up to 90 min in order to restore arterial patency is associated with equivalent outcomes. The routine use of coronary stents in patients with acute MI is associated with a restenosis rate of 17 % and a 6-month event-free survival of 83–95 % [358, 501, 502, 517, 518]. Drug-eluting stents further improve outcomes, reducing the recurrent event rate to 7–9 % [516, 519].
Routine angioplasty after full-dose thrombolytic therapy has been associated with an increased risk of complications due to rethrombosis, bleeding, and coronary artery intramural hematoma that could produce abrupt closure [272, 275, 520]. However, with the use of stents and glycoprotein IIb/IIIa antagonists, not only is the increased complication risk associated with PTCA abolished, but the risk of death, reinfarction, and repeat hospitalization for revascularization is substantially reduced [521–523]. Direct thrombin antagonists have compared favorably with the combination of heparin and a GP IIb/IIIa antagonist, possibly decreasing bleeding after the procedure [524]. After failed thrombolysis or in patients with cardiogenic shock who have received thrombolysis, invasive investigation with intention for stent revascularization if possible increases myocardial salvage and reduces the risk of death, heart failure, or reinfarction [499, 511, 525, 526]. Therefore, early angiography and possible percutaneous revascularization of the culprit lesion after thrombolytic therapy for acute MI should be strongly considered [527].
Each approach to the treatment of MI, full-dose thrombolytic therapy, and primary stent-PCR has specific strengths and weaknesses. Thrombolysis may be introduced rapidly on confirming ongoing myocardial injury and may be made widely available. Percutaneous coronary revascularization requires mobilizing a specially trained team and sophisticated equipment that may produce delays in effecting treatment. However, in patients destined for emergency PCR, the administration of thrombolytics in addition to glycoprotein IIb/IIIa antagonists before the procedure offers no advantage over the use of glycoprotein IIb/IIIa antagonists alone [528].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


